当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
B2M gene expression shapes the immune landscape of lung adenocarcinoma and determines the response to immunotherapy
Immunology ( IF 4.9 ) Pub Date : 2021-06-11 , DOI: 10.1111/imm.13384 Yu Zhao 1 , Yuejiao Cao 2 , Yiqi Chen 1 , Lei Wu 1 , Hua Hang 3 , Chenxia Jiang 3 , Xiaorong Zhou 1, 4
Immunology ( IF 4.9 ) Pub Date : 2021-06-11 , DOI: 10.1111/imm.13384 Yu Zhao 1 , Yuejiao Cao 2 , Yiqi Chen 1 , Lei Wu 1 , Hua Hang 3 , Chenxia Jiang 3 , Xiaorong Zhou 1, 4
Affiliation
|
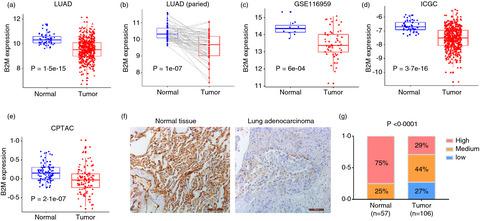
Loss of the B2M gene is associated with tumour immune escape and resistance to immunotherapy. However, genetic alterations of the B2M gene are rare. We performed an integrative analysis of the mutational and transcriptional profiles of large cohorts of non-small-cell lung cancer (NSCLC) patients and found that epigenetic downregulation of B2M is common. B2M-low tumours exhibit a suppressive immune microenvironment characterized by reduced infiltration of immune cells of various lineages; in B2M-high tumours, more T and natural killer cells are present, but their activities are constrained by immune checkpoint molecules, indicating the diverse mechanisms of immune evasion. High levels of B2M mRNA, but not PD-L1, are correlated with an enhanced response to PD-1-based immunotherapy, suggesting its value for immunotherapy response prediction in solid tumours. Notably, a high tumour mutation burden (TMB) is associated with low B2M expression, which may explain the poor predictive value of the TMB in some situations. In syngeneic mouse models, genetic ablation of B2M in tumour cells causes resistance to PD-1-based immunotherapy, and B2M knockdown also diminishes the therapeutic efficacy. Moreover, forced expression of B2M in tumour models improves the response to immunotherapy, suggesting that B2M levels have significant impacts on treatment outcomes. Finally, we provide insight into the roles of transcription factors and KRAS mutations in B2M expression and the anticancer immune response. In conclusion, genetic and epigenetic regulation of B2M fundamentally shapes the NSCLC immune microenvironment and may determine the response to checkpoint blockade-based immunotherapy.
中文翻译:

B2M 基因表达塑造了肺腺癌的免疫景观并决定了对免疫治疗的反应
B2M基因的缺失与肿瘤免疫逃逸和对免疫治疗的抵抗有关。然而,B2M基因的遗传改变很少见。我们对大型非小细胞肺癌 (NSCLC) 患者队列的突变和转录谱进行了综合分析,发现B2M的表观遗传下调常见。B2M-low 肿瘤表现出抑制性免疫微环境,其特征是各种谱系免疫细胞的浸润减少;在 B2M 高的肿瘤中,存在更多的 T 细胞和自然杀伤细胞,但它们的活动受到免疫检查点分子的限制,表明免疫逃避的多种机制。高水平的 B2M mRNA(但与 PD-L1 无关)与对基于 PD-1 的免疫疗法的增强反应相关,表明其在实体瘤中免疫治疗反应预测的价值。值得注意的是,高肿瘤突变负荷 (TMB) 与低 B2M 表达相关,这可能解释了 TMB 在某些情况下的预测价值低。在同基因小鼠模型中,B2M的基因消融在肿瘤细胞中导致对基于 PD-1 的免疫疗法的抗性,并且 B2M 敲低也会降低治疗效果。此外,B2M 在肿瘤模型中的强制表达提高了对免疫治疗的反应,这表明 B2M 水平对治疗结果有显着影响。最后,我们深入了解了转录因子和KRAS突变在 B2M 表达和抗癌免疫反应中的作用。总之,B2M 的遗传和表观遗传调控从根本上塑造了 NSCLC 免疫微环境,并可能决定对基于检查点阻断的免疫疗法的反应。
更新日期:2021-06-11
中文翻译:

B2M 基因表达塑造了肺腺癌的免疫景观并决定了对免疫治疗的反应
B2M基因的缺失与肿瘤免疫逃逸和对免疫治疗的抵抗有关。然而,B2M基因的遗传改变很少见。我们对大型非小细胞肺癌 (NSCLC) 患者队列的突变和转录谱进行了综合分析,发现B2M的表观遗传下调常见。B2M-low 肿瘤表现出抑制性免疫微环境,其特征是各种谱系免疫细胞的浸润减少;在 B2M 高的肿瘤中,存在更多的 T 细胞和自然杀伤细胞,但它们的活动受到免疫检查点分子的限制,表明免疫逃避的多种机制。高水平的 B2M mRNA(但与 PD-L1 无关)与对基于 PD-1 的免疫疗法的增强反应相关,表明其在实体瘤中免疫治疗反应预测的价值。值得注意的是,高肿瘤突变负荷 (TMB) 与低 B2M 表达相关,这可能解释了 TMB 在某些情况下的预测价值低。在同基因小鼠模型中,B2M的基因消融在肿瘤细胞中导致对基于 PD-1 的免疫疗法的抗性,并且 B2M 敲低也会降低治疗效果。此外,B2M 在肿瘤模型中的强制表达提高了对免疫治疗的反应,这表明 B2M 水平对治疗结果有显着影响。最后,我们深入了解了转录因子和KRAS突变在 B2M 表达和抗癌免疫反应中的作用。总之,B2M 的遗传和表观遗传调控从根本上塑造了 NSCLC 免疫微环境,并可能决定对基于检查点阻断的免疫疗法的反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号