当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Antibacterial Activity of Propylamycin Derivatives Functionalized at the 5′′- and Other Positions with a View to Overcoming Resistance Due to Aminoglycoside Modifying Enzymes
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-06-11 , DOI: 10.1021/acsinfecdis.1c00158 Dimitrijs Lubriks 1 , Rimants Zogota 1 , Vikram A Sarpe 2, 3 , Takahiko Matsushita 4 , Girish C Sati 4 , Klara Haldimann 5 , Marina Gysin 5 , Erik C Böttger 5 , Andrea Vasella 6 , Edgars Suna 1 , Sven N Hobbie 5 , David Crich 2, 3, 4, 7
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-06-11 , DOI: 10.1021/acsinfecdis.1c00158 Dimitrijs Lubriks 1 , Rimants Zogota 1 , Vikram A Sarpe 2, 3 , Takahiko Matsushita 4 , Girish C Sati 4 , Klara Haldimann 5 , Marina Gysin 5 , Erik C Böttger 5 , Andrea Vasella 6 , Edgars Suna 1 , Sven N Hobbie 5 , David Crich 2, 3, 4, 7
Affiliation
|
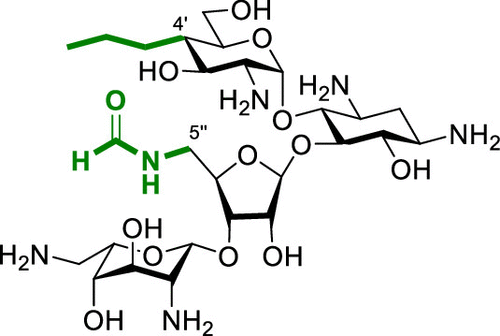
Propylamycin (4′-deoxy-4′-propylparomomycin) is a next generation aminoglycoside antibiotic that displays increased antibacterial potency over the parent, coupled with reduced susceptibility to resistance determinants and reduced ototoxicity in the guinea pig model. Propylamycin nevertheless is inactivated by APH(3′)-Ia, a specific aminoglycoside phosphotransferase isozyme that acts on the primary hydroxy group of the ribofuranosyl moiety (at the 5′′-position). To overcome this problem, we have prepared and studied the antibacterial and antiribosomal activity of various propylamycin derivatives carrying amino or substituted amino groups at the 5′′-position in place of the vulnerable hydroxy group. We find that the introduction of an additional basic amino group at this position, while overcoming the action of the aminoglycoside phosphoryltransferase isozymes acting at the 5′′-position as anticipated, results in a significant drop in selectivity for the bacterial over the eukaryotic ribosomes that is predictive of increased ototoxicity. In contrast, 5′′-deoxy-5′′-formamidopropylamycin retains the excellent across-the-board levels of antibacterial activity of propylamycin itself, while circumventing the action of the offending aminoglycoside phosphotransferase isozymes and affording even greater selectivity for the bacterial over the eukaryotic ribosomes. Other modifications to address the susceptibility of propylamycin to the APH(3′)-Ia isozyme including deoxygenation at the 3′-position and incorporation of a 6′,5′′-bis(hydroxyethylamino) modification offer no particular advantage.
中文翻译:

以克服氨基糖苷类修饰酶耐药为目的,5''-等位功能化丙霉素衍生物的合成及抗菌活性
Propylamycin (4'-deoxy-4'-propylparomomycin) 是下一代氨基糖苷类抗生素,在豚鼠模型中表现出比亲本更高的抗菌效力,同时对耐药决定因素的敏感性降低和耳毒性降低。然而丙霉素被 APH(3')-Ia 灭活,APH(3')-Ia 是一种特定的氨基糖苷磷酸转移酶同工酶,作用于呋喃核糖基部分的伯羟基(在 5''-位)。为了克服这个问题,我们制备并研究了各种丙霉素衍生物的抗菌和抗核糖体活性,这些衍生物在 5''-位带有氨基或取代的氨基以代替易受伤害的羟基。我们发现在这个位置引入一个额外的碱性氨基,虽然如预期的那样克服了作用于 5'' 位的氨基糖苷类磷酸转移酶同工酶的作用,但导致细菌对真核核糖体的选择性显着下降,这预示着耳毒性增加。相比之下,5''-deoxy-5''-formamidopropylamycin 保留了丙霉素本身优异的全面抗菌活性水平,同时规避了有问题的氨基糖苷磷酸转移酶同工酶的作用,并为细菌提供了更大的选择性。真核核糖体。解决丙霉素对 APH(3')-Ia 同工酶敏感性的其他修饰,包括在 3'-位脱氧和掺入 6',5''-双(羟乙基氨基)修饰没有提供特别优势。
更新日期:2021-08-13
中文翻译:

以克服氨基糖苷类修饰酶耐药为目的,5''-等位功能化丙霉素衍生物的合成及抗菌活性
Propylamycin (4'-deoxy-4'-propylparomomycin) 是下一代氨基糖苷类抗生素,在豚鼠模型中表现出比亲本更高的抗菌效力,同时对耐药决定因素的敏感性降低和耳毒性降低。然而丙霉素被 APH(3')-Ia 灭活,APH(3')-Ia 是一种特定的氨基糖苷磷酸转移酶同工酶,作用于呋喃核糖基部分的伯羟基(在 5''-位)。为了克服这个问题,我们制备并研究了各种丙霉素衍生物的抗菌和抗核糖体活性,这些衍生物在 5''-位带有氨基或取代的氨基以代替易受伤害的羟基。我们发现在这个位置引入一个额外的碱性氨基,虽然如预期的那样克服了作用于 5'' 位的氨基糖苷类磷酸转移酶同工酶的作用,但导致细菌对真核核糖体的选择性显着下降,这预示着耳毒性增加。相比之下,5''-deoxy-5''-formamidopropylamycin 保留了丙霉素本身优异的全面抗菌活性水平,同时规避了有问题的氨基糖苷磷酸转移酶同工酶的作用,并为细菌提供了更大的选择性。真核核糖体。解决丙霉素对 APH(3')-Ia 同工酶敏感性的其他修饰,包括在 3'-位脱氧和掺入 6',5''-双(羟乙基氨基)修饰没有提供特别优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号