当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic Mimics of Native Siderophores Disrupt Iron Trafficking in Acinetobacter baumannii
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-06-10 , DOI: 10.1021/acsinfecdis.1c00119 Tabbetha J Bohac 1 , Luting Fang 1 , Victoria S Banas 1 , Daryl E Giblin 1 , Timothy A Wencewicz 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-06-10 , DOI: 10.1021/acsinfecdis.1c00119 Tabbetha J Bohac 1 , Luting Fang 1 , Victoria S Banas 1 , Daryl E Giblin 1 , Timothy A Wencewicz 1
Affiliation
|
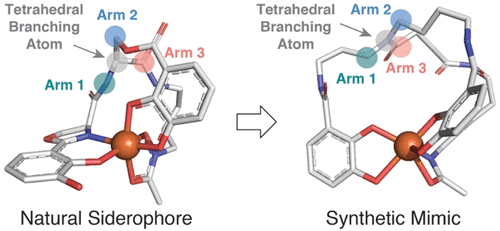
Many pathogenic bacteria biosynthesize and excrete small molecule metallophores, known as siderophores, that are used to extract ferric iron from host sources to satisfy nutritional need. Native siderophores are often structurally complex multidentate chelators that selectively form high-affinity octahedral ferric iron complexes with defined chirality recognizable by cognate protein receptors displayed on the bacterial cell surface. Simplified achiral analogues can serve as synthetically tractable siderophore mimics with potential utility as chemical probes and therapeutic agents to better understand and treat bacterial infections, respectively. Here, we demonstrate that synthetic spermidine-derived mixed ligand bis-catecholate monohydroxamate siderophores (compounds 1–3) are versatile structural and biomimetic analogues of two native siderophores, acinetobactin and fimsbactin, produced by Acinetobacter baumannii, a multidrug-resistant Gram-negative human pathogen. The metal-free and ferric iron complexes of the synthetic siderophores are growth-promoting agents of A. baumannii, while the Ga(III)-complexes are potent growth inhibitors of A. baumannii with MIC values <1 μM. The synthetic siderophores compete with native siderophores for uptake in A. baumannii and maintain comparable apparent binding affinities for ferric iron (KFe) and the siderophore-binding protein BauB (Kd). Our findings provide new insight to guide the structural fine-tuning of these compounds as siderophore-based therapeutics targeting pathogenic strains of A. baumannii.
中文翻译:

天然铁载体的合成模拟物破坏鲍曼不动杆菌中的铁转运
许多病原菌生物合成和排泄小分子金属载体,称为铁载体,用于从宿主来源提取三价铁以满足营养需求。天然铁载体通常是结构复杂的多齿螯合剂,它们选择性地形成具有确定手性的高亲和力八面体三价铁络合物,可通过细菌细胞表面上显示的同源蛋白受体识别。简化的非手性类似物可以作为合成易处理的铁载体模拟物,具有作为化学探针和治疗剂的潜在用途,可以分别更好地了解和治疗细菌感染。在这里,我们证明,亚精胺合成衍生的混合配位体双-儿茶酚monohydroxamate铁载体(化合物1 - 3) 是由多重耐药革兰氏阴性人类病原体鲍曼不动杆菌( Acinetobacter baumannii)生产的两种天然铁载体不动杆菌素和纤毛菌素的多功能结构和仿生类似物。合成铁载体的不含金属和三价铁配合物的生长促进剂鲍曼不动杆菌,而镓(III)-complexes是的有效生长抑制剂鲍曼不动杆菌与MIC值<1微米。合成铁载体与天然铁载体竞争在鲍曼不动杆菌中的摄取,并保持对三价铁 ( K Fe ) 和铁载体结合蛋白 BauB ( K d)。我们的发现为指导这些化合物的结构微调提供了新的见解,作为靶向鲍曼不动杆菌致病菌株的铁载体疗法。
更新日期:2021-08-13
中文翻译:

天然铁载体的合成模拟物破坏鲍曼不动杆菌中的铁转运
许多病原菌生物合成和排泄小分子金属载体,称为铁载体,用于从宿主来源提取三价铁以满足营养需求。天然铁载体通常是结构复杂的多齿螯合剂,它们选择性地形成具有确定手性的高亲和力八面体三价铁络合物,可通过细菌细胞表面上显示的同源蛋白受体识别。简化的非手性类似物可以作为合成易处理的铁载体模拟物,具有作为化学探针和治疗剂的潜在用途,可以分别更好地了解和治疗细菌感染。在这里,我们证明,亚精胺合成衍生的混合配位体双-儿茶酚monohydroxamate铁载体(化合物1 - 3) 是由多重耐药革兰氏阴性人类病原体鲍曼不动杆菌( Acinetobacter baumannii)生产的两种天然铁载体不动杆菌素和纤毛菌素的多功能结构和仿生类似物。合成铁载体的不含金属和三价铁配合物的生长促进剂鲍曼不动杆菌,而镓(III)-complexes是的有效生长抑制剂鲍曼不动杆菌与MIC值<1微米。合成铁载体与天然铁载体竞争在鲍曼不动杆菌中的摄取,并保持对三价铁 ( K Fe ) 和铁载体结合蛋白 BauB ( K d)。我们的发现为指导这些化合物的结构微调提供了新的见解,作为靶向鲍曼不动杆菌致病菌株的铁载体疗法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号