Critical Reviews in Oncology/Hematology ( IF 5.5 ) Pub Date : 2021-06-11 , DOI: 10.1016/j.critrevonc.2021.103393 Yuzhong Chen 1 , Shaodi Wen 1 , Yuan Wu 1 , Lin Shi 1 , Xiaoyue Xu 1 , Bo Shen 1
|
Objective
We conducted a meta-analysis to synthesize the results of published randomized controlled trials conducted to evaluate the efficacy and safety of epidermal growth factor receptor - tyrosine kinase inhibitors (EGFR-TKIs) combined with chemotherapy or antiangiogenic therapy.
Methods
PubMed, EMBASE, Cochrane Library and ClinicalTrials.gov databases were searched and literatures from international conferences were read to identify eligible studies. The primary endpoints were objective response rate (ORR) and progression free survival (PFS). The secondary endpoints were disease control rate (DCR), overall survival (OS) and treatment-emergent adverse events (TEAEs).
Results
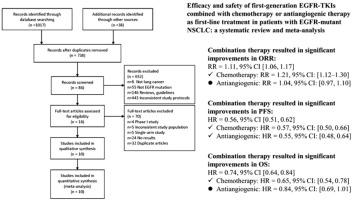
10 studies, all on first-generation EGFR-TKI combination therapy, involving 2367 patients were included. Combination therapy resulted in significant improvements in ORR (RR: 1.11, 95% CI: 1.06–1.17, P < 0.001), DCR (RR: 1.03, 95% CI: 1.01–1.05, P = 0.007), PFS (HR: 0.56, 95% CI: 0.51–0.62, P < 0.001), OS (HR: 0.74, 95% CI: 0.64–0.84, P = 0.002) over monotherapy. This improvement was more apparent in the EGFR-TKIs combination chemotherapy group, and indirect comparisons revealed that EGFR-TKIs combined with chemotherapy appeared to be superior to combined with antiangiogenic therapy in ORR (RR: 1.19, 95% CI: 1.07–1.32), DCR (RR: 1.04, 95% CI: 1.02–1.08), and OS (HR: 0.79, 95% CI: 0.66−0.96). Of additional concern is the increased incidence of TEAEs in combination therapy.
Conclusion
As a first-line treatment for patients with EGFR-mutated advanced non-small cell lung cancer (NSCLC), first-generation EGFR-TKIs combined with chemotherapy or antiangiogenic therapy was associated with significant improvement in ORR, DCR, PFS and OS compared with monotherapy.
中文翻译:

第一代表皮生长因子受体 (EGFR) 酪氨酸激酶抑制剂 (TKIs) 联合化疗或抗血管生成治疗作为 EGFR 突变非小细胞肺癌患者一线治疗的疗效和安全性:系统评价和荟萃分析分析
客观的
我们进行了一项荟萃分析来综合已发表的随机对照试验的结果,这些试验旨在评估表皮生长因子受体 - 酪氨酸激酶抑制剂 (EGFR-TKI) 联合化疗或抗血管生成治疗的疗效和安全性。
方法
检索了 PubMed、EMBASE、Cochrane 图书馆和 ClinicalTrials.gov 数据库,并阅读了国际会议的文献以确定符合条件的研究。主要终点是客观缓解率(ORR)和无进展生存期(PFS)。次要终点是疾病控制率(DCR)、总生存期(OS)和治疗出现的不良事件(TEAE)。
结果
纳入第一代EGFR-TKI联合治疗10项研究,共2367例患者。联合治疗显着改善了 ORR(RR:1.11,95% CI:1.06-1.17,P < 0.001)、DCR(RR:1.03,95% CI:1.01-1.05,P = 0.007)、PFS(HR:0.56) , 95% CI: 0.51–0.62, P < 0.001), OS (HR: 0.74, 95% CI: 0.64–0.84, P = 0.002) 优于单药治疗。这种改善在 EGFR-TKIs 联合化疗组中更为明显,间接比较显示 EGFR-TKIs 联合化疗在 ORR 方面似乎优于联合抗血管生成治疗(RR:1.19,95% CI:1.07-1.32), DCR(RR:1.04,95% CI:1.02-1.08)和 OS(HR:0.79,95% CI:0.66-0.96)。另一个令人担忧的是联合治疗中 TEAE 的发生率增加。
结论
作为EGFR突变的晚期非小细胞肺癌(NSCLC)患者的一线治疗,第一代EGFR-TKIs联合化疗或抗血管生成治疗与ORR、DCR、PFS和OS的显着改善相关。单一疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号