当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphorylation of trans-active response DNA-binding protein-of 43 kDa promotes its cytoplasmic aggregation and modulates its function in tau mRNA stability and exon 10 alternative splicing
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2021-06-09 , DOI: 10.1111/jnc.15450 Ruozhen Wu 1, 2 , Dingwei Zhou 1, 2 , Xin Shen 1 , Feng Chen 1 , Fei Liu 2 , Jianlan Gu 1, 2
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2021-06-09 , DOI: 10.1111/jnc.15450 Ruozhen Wu 1, 2 , Dingwei Zhou 1, 2 , Xin Shen 1 , Feng Chen 1 , Fei Liu 2 , Jianlan Gu 1, 2
Affiliation
|
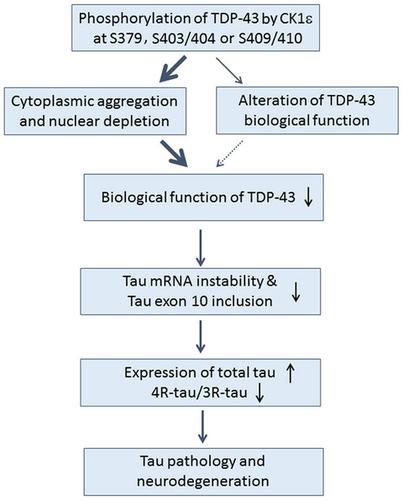
Trans-active response DNA-binding protein of 43 kDa (TDP-43) promotes tau mRNA instability and tau exon 10 inclusion. Aggregation of phosphorylated TDP-43 is associated with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration. Casein kinase 1ε (CK1ε) phosphorylates TDP-43 at multiple sites, enhances its cytoplasmic aggregation, and modulates its function in tau mRNA processing. To determine roles of TDP-43 site-specific phosphorylation in its localization, aggregation, and function in tau mRNA processing, TDP-43 was mutated to alanine or aspartic acid at Ser379, Ser403/404, or Ser409/410 to block or mimic phosphorylation. Site-specific phosphorylation of TDP-43 and its mutants by CK1ε was studied in vitro and in cultured cells. Cytoplasmic and nuclear TDP-43 and phospho-TDP-43 were analyzed by western blots. Aggregation of TDP-43 was assessed by immunostaining and level of radioimmunoprecipitation assay buffer-insoluble TDP-43. Green florescent protein tailed with tau 3′-untranslated region and mini-tau gene pCI/SI9-LI10 were used to study tau mRNA stability and alternative splicing of tau exon 10. We found that phospho-blocking mutations of TDP-43 at Ser379, Ser403/404, or Ser409/410 were not effectively phosphorylated by CK1ε. Compared with TDP-43, higher level of phosphorylated TDP-43 in the cytoplasm was observed. Phospho-mimicking mutations at these sites enhanced cytoplasmic aggregation of TDP-43. Green florescent protein expression was not inhibited by phospho-blocking mutants of TDP-43, but tau exon 10 inclusion was further enhanced by phospho-blocking mutations at Ser379 and Ser403/404. Phosphorylation of TDP-43 at Ser379, Ser403/404, or Ser409/410 primes its phosphorylation by CK1ε, promotes TDP-43 cytoplasmic aggregation, and modulates its function in tau mRNA processing in site-specific manner.
中文翻译:

43 kDa 的反式反应 DNA 结合蛋白的磷酸化促进其细胞质聚集并调节其在 tau mRNA 稳定性和外显子 10 可变剪接中的功能
43 kDa 的反式反应 DNA 结合蛋白 (TDP-43) 促进 tau mRNA 不稳定性和 tau 外显子 10 包含。磷酸化 TDP-43 的聚集与肌萎缩侧索硬化 (ALS) 和额颞叶变性有关。酪蛋白激酶 1ε (CK1ε) 在多个位点磷酸化 TDP-43,增强其细胞质聚集,并调节其在 tau mRNA 加工中的功能。为了确定 TDP-43 位点特异性磷酸化在 tau mRNA 加工中的定位、聚集和功能中的作用,TDP-43 在 Ser379、Ser403/404 或 Ser409/410 处突变为丙氨酸或天冬氨酸以阻断或模拟磷酸化. 在体外和培养细胞中研究了 CK1ε 对 TDP-43 及其突变体的位点特异性磷酸化。通过蛋白质印迹分析细胞质和核 TDP-43 和磷酸化 TDP-43。TDP-43 的聚集通过免疫染色和放射免疫沉淀测定缓冲液不溶性 TDP-43 的水平来评估。尾端带有 tau 3'-非翻译区和 mini-tau 基因 pCI/SI9-LI10 的绿色荧光蛋白用于研究 tau mRNA 稳定性和 tau 外显子 10 的可变剪接。我们发现 TDP-43 在 Ser379、 Ser403/404 或 Ser409/410 没有被 CK1ε 有效磷酸化。与 TDP-43 相比,在细胞质中观察到更高水平的磷酸化 TDP-43。这些位点的磷模拟突变增强了 TDP-43 的细胞质聚集。TDP-43 的磷酸化阻断突变体不会抑制绿色荧光蛋白的表达,但 Ser379 和 Ser403/404 的磷酸化阻断突变进一步增强了 tau 外显子 10 的包含。TDP-43 在 Ser379、Ser403/404、
更新日期:2021-08-12
中文翻译:

43 kDa 的反式反应 DNA 结合蛋白的磷酸化促进其细胞质聚集并调节其在 tau mRNA 稳定性和外显子 10 可变剪接中的功能
43 kDa 的反式反应 DNA 结合蛋白 (TDP-43) 促进 tau mRNA 不稳定性和 tau 外显子 10 包含。磷酸化 TDP-43 的聚集与肌萎缩侧索硬化 (ALS) 和额颞叶变性有关。酪蛋白激酶 1ε (CK1ε) 在多个位点磷酸化 TDP-43,增强其细胞质聚集,并调节其在 tau mRNA 加工中的功能。为了确定 TDP-43 位点特异性磷酸化在 tau mRNA 加工中的定位、聚集和功能中的作用,TDP-43 在 Ser379、Ser403/404 或 Ser409/410 处突变为丙氨酸或天冬氨酸以阻断或模拟磷酸化. 在体外和培养细胞中研究了 CK1ε 对 TDP-43 及其突变体的位点特异性磷酸化。通过蛋白质印迹分析细胞质和核 TDP-43 和磷酸化 TDP-43。TDP-43 的聚集通过免疫染色和放射免疫沉淀测定缓冲液不溶性 TDP-43 的水平来评估。尾端带有 tau 3'-非翻译区和 mini-tau 基因 pCI/SI9-LI10 的绿色荧光蛋白用于研究 tau mRNA 稳定性和 tau 外显子 10 的可变剪接。我们发现 TDP-43 在 Ser379、 Ser403/404 或 Ser409/410 没有被 CK1ε 有效磷酸化。与 TDP-43 相比,在细胞质中观察到更高水平的磷酸化 TDP-43。这些位点的磷模拟突变增强了 TDP-43 的细胞质聚集。TDP-43 的磷酸化阻断突变体不会抑制绿色荧光蛋白的表达,但 Ser379 和 Ser403/404 的磷酸化阻断突变进一步增强了 tau 外显子 10 的包含。TDP-43 在 Ser379、Ser403/404、











































 京公网安备 11010802027423号
京公网安备 11010802027423号