当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proteolysis of γ-tubulin small complex proteins is mediated by the ubiquitin-proteasome system
FEBS Letters ( IF 3.0 ) Pub Date : 2021-06-09 , DOI: 10.1002/1873-3468.14146 Can Yin 1 , Edna S W Lui 1 , Taolue Jiang 1 , Robert Z Qi 1
FEBS Letters ( IF 3.0 ) Pub Date : 2021-06-09 , DOI: 10.1002/1873-3468.14146 Can Yin 1 , Edna S W Lui 1 , Taolue Jiang 1 , Robert Z Qi 1
Affiliation
|
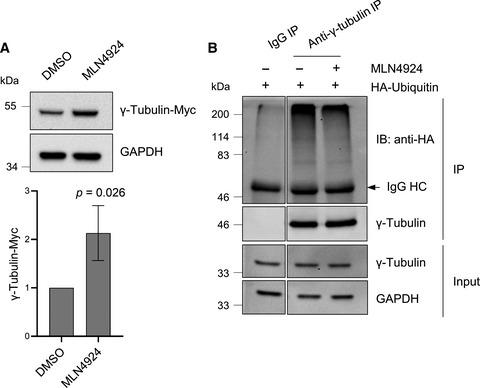
Microtubule nucleation is mainly mediated by the γ-tubulin ring complex (γTuRC), whose core components are γ-tubulin and γ-tubulin complex proteins GCP2–6. A substantial fraction of γ-tubulin also exists with GCP2 and GCP3 in a tetramer called the γ-tubulin small complex (γTuSC). To date, the mechanisms underlying the turnover of γ-tubulin and GCPs have remained unclear. Here, we show that γ-tubulin, GCP2, and GCP3 are proteolyzed by the ubiquitin-proteasome system, and we identify cullin 1, cullin 4A, and cullin 4B as the E3 ligases that mediate the ubiquitination and, consequently, the degradation of γ-tubulin. Notably, we found that γTuSC disassembly promotes the degradation of γ-tubulin, GCP2, and GCP3, which indicates a role for γTuSCs in the stabilization of its components.
中文翻译:

γ-微管蛋白小复合蛋白的蛋白水解由泛素-蛋白酶体系统介导
微管成核主要由γ-微管蛋白环复合物(γTuRC)介导,其核心成分是γ-微管蛋白和γ-微管蛋白复合蛋白GCP2-6。大部分 γ-微管蛋白也与 GCP2 和 GCP3 一起存在于称为 γ-微管蛋白小复合物 (γTuSC) 的四聚体中。迄今为止,γ-微管蛋白和 GCP 转换的潜在机制仍不清楚。在这里,我们表明 γ-微管蛋白、GCP2 和 GCP3 被泛素-蛋白酶体系统蛋白水解,并且我们将 cullin 1、cullin 4A 和 cullin 4B 鉴定为介导泛素化的 E3 连接酶,从而介导 γ 的降解-微管蛋白。值得注意的是,我们发现 γTuSC 的分解促进了 γ-微管蛋白、GCP2 和 GCP3 的降解,这表明 γTuSCs 在稳定其组分中的作用。
更新日期:2021-08-09
中文翻译:

γ-微管蛋白小复合蛋白的蛋白水解由泛素-蛋白酶体系统介导
微管成核主要由γ-微管蛋白环复合物(γTuRC)介导,其核心成分是γ-微管蛋白和γ-微管蛋白复合蛋白GCP2-6。大部分 γ-微管蛋白也与 GCP2 和 GCP3 一起存在于称为 γ-微管蛋白小复合物 (γTuSC) 的四聚体中。迄今为止,γ-微管蛋白和 GCP 转换的潜在机制仍不清楚。在这里,我们表明 γ-微管蛋白、GCP2 和 GCP3 被泛素-蛋白酶体系统蛋白水解,并且我们将 cullin 1、cullin 4A 和 cullin 4B 鉴定为介导泛素化的 E3 连接酶,从而介导 γ 的降解-微管蛋白。值得注意的是,我们发现 γTuSC 的分解促进了 γ-微管蛋白、GCP2 和 GCP3 的降解,这表明 γTuSCs 在稳定其组分中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号