Chem ( IF 19.1 ) Pub Date : 2021-06-09 , DOI: 10.1016/j.chempr.2021.05.002 Fabian Ebner 1 , Lutz Greb 1
|
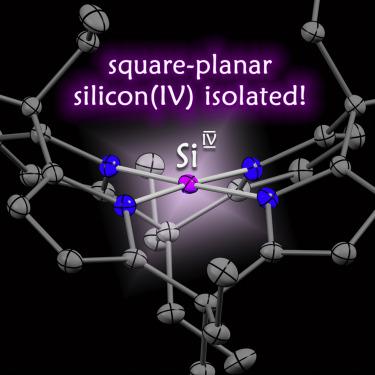
The structure and reactivity of silicon(IV), the second most abundant element in our Earth's crust, is determined by its invariant tetrahedral coordination geometry. Silicon(IV) with a square-planar configuration (ptSiIV) represents a transition state. Quantum theory supported the feasibility of stabilizing ptSiIV by structural constraint, but its isolation has not been achieved yet. Here, we present the synthesis and full characterization of the first square-planar coordinated silicon(IV). The planarity provokes an extremely low-lying unoccupied molecular orbital that induces unusual silicon redox chemistry and CH-agostic interactions. The small separation of the frontier molecular orbitals enables visible-light ligand-element charge transfer and bond-activation reactivity. Previously, such characteristics have been reserved for d-block metals or low-valent p-block elements. Planarization transfers them, for the first time, to a p-block element in the normal valence state.
中文翻译:

方形平面硅 (IV) 的可分离结晶复合物
硅 (IV) 是地壳中含量第二丰富的元素,其结构和反应性由其不变的四面体配位几何形状决定。具有方形平面配置 ( ptSi IV ) 的硅 (IV)代表过渡态。量子理论支持稳定ptSi IV的可行性受结构约束,但尚未实现其隔离。在这里,我们展示了第一个方形平面配位硅 (IV) 的合成和完整表征。平面性引发了一个极低的未占据分子轨道,从而引发了不寻常的硅氧化还原化学和 CH-积极相互作用。前沿分子轨道的小间距使可见光配体元素电荷转移和键活化反应成为可能。以前,此类特性已保留用于 d 区金属或低价 p 区元素。平面化第一次将它们转移到正常价态的 p 块元素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号