当前位置:
X-MOL 学术
›
Biopolymers
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyrrolidinyl peptide nucleic acids bearing hydroxy-modified cyclobutane building blocks: Synthesis and binding properties
Biopolymers ( IF 3.2 ) Pub Date : 2021-06-08 , DOI: 10.1002/bip.23459 Boonsong Ditmangklo 1 , Wantanee Sittiwong 2 , Thomas Boddaert 3 , Tirayut Vilaivan 1 , David J Aitken 3
Biopolymers ( IF 3.2 ) Pub Date : 2021-06-08 , DOI: 10.1002/bip.23459 Boonsong Ditmangklo 1 , Wantanee Sittiwong 2 , Thomas Boddaert 3 , Tirayut Vilaivan 1 , David J Aitken 3
Affiliation
|
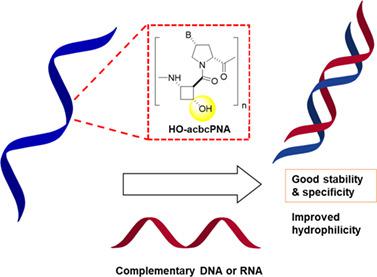
The conformationally constrained pyrrolidinyl PNA with a dipeptide consisting of an alternating nucleobase-modified D-proline and a cyclic β-amino acid “spacer” exhibited improved nucleic acid binding properties compared to the original PNA. The pyrrolidinyl PNA with the four-membered ring spacer (1S,2S)-2-aminocyclobutanecarboxylic acid (acbcPNA) are among the best performed members of the pyrrolidinyl PNA family. However, these PNA suffer some limitations such as aqueous solubility and non-specific interactions due to their extreme hydrophobicity. In the present work, a hydroxy group is introduced onto the cyclobutane ring spacer of the acbcPNA with the aim of decreasing its hydrophobicity. To this end, a Fmoc/tBu ether-protected 4-hydroxy-2-aminocyclobutanecarboxylic acid building block was synthesized and resolved by chiral HPLC. Each enantiomer was used to synthesize the hydroxy-modified acbcPNA employing Fmoc solid-phase peptide synthesis. DNA/RNA binding studies indicated that the introduction of the hydroxy group to the acbcPNA decreases the binding affinity toward complementary DNA and RNA while maintaining the sequence and directional specificity of unmodified acbcPNA. The hydrophobicity of the hydroxy-modified acbcPNA decreased with the number of hydroxy groups added as indicated by the decrease in the logP values. Only two modifications were sufficient to decrease the logP by an order of magnitude without excessively lowering the binding affinity nor the specificity. This work thus demonstrated that the specific structural modifications for this type of PNA model can be performed in a modular fashion, which paves the way toward the future realization of improving hydrophilicity and nucleic acid binding affinity as well as specificity.
中文翻译:

带有羟基修饰的环丁烷结构单元的吡咯烷基肽核酸:合成和结合特性
与原始 PNA 相比,具有由交替的核碱基修饰的 D-脯氨酸和环状 β-氨基酸“间隔区”组成的二肽的构象受限的吡咯烷基 PNA 表现出改进的核酸结合特性。具有四元环间隔子 (1 S ,2 S )-2-氨基环丁烷羧酸 (acbcPNA) 的吡咯烷基 PNA 是吡咯烷基 PNA 家族中性能最好的成员之一。然而,这些 PNA 由于其极端疏水性而受到一些限制,例如水溶性和非特异性相互作用。在目前的工作中,将羟基引入到 acbcPNA 的环丁烷环间隔基上,目的是降低其疏水性。为此,一个 Fmoc/ t合成了丁醚保护的 4-羟基-2-氨基环丁烷羧酸结构单元,并通过手性 HPLC 分离。每种对映异构体用于合成羟基修饰的 acbcPNA,采用 Fmoc 固相肽合成。DNA/RNA 结合研究表明,将羟基引入 acbcPNA 会降低对互补 DNA 和 RNA 的结合亲和力,同时保持未修饰的 acbcPNA 的序列和方向特异性。羟基修饰的 acbcPNA 的疏水性随着添加的羟基的数量而降低,如 logP 值的降低所示。只有两个修改足以将 logP 降低一个数量级,而不会过度降低结合亲和力或特异性。
更新日期:2021-06-08
中文翻译:

带有羟基修饰的环丁烷结构单元的吡咯烷基肽核酸:合成和结合特性
与原始 PNA 相比,具有由交替的核碱基修饰的 D-脯氨酸和环状 β-氨基酸“间隔区”组成的二肽的构象受限的吡咯烷基 PNA 表现出改进的核酸结合特性。具有四元环间隔子 (1 S ,2 S )-2-氨基环丁烷羧酸 (acbcPNA) 的吡咯烷基 PNA 是吡咯烷基 PNA 家族中性能最好的成员之一。然而,这些 PNA 由于其极端疏水性而受到一些限制,例如水溶性和非特异性相互作用。在目前的工作中,将羟基引入到 acbcPNA 的环丁烷环间隔基上,目的是降低其疏水性。为此,一个 Fmoc/ t合成了丁醚保护的 4-羟基-2-氨基环丁烷羧酸结构单元,并通过手性 HPLC 分离。每种对映异构体用于合成羟基修饰的 acbcPNA,采用 Fmoc 固相肽合成。DNA/RNA 结合研究表明,将羟基引入 acbcPNA 会降低对互补 DNA 和 RNA 的结合亲和力,同时保持未修饰的 acbcPNA 的序列和方向特异性。羟基修饰的 acbcPNA 的疏水性随着添加的羟基的数量而降低,如 logP 值的降低所示。只有两个修改足以将 logP 降低一个数量级,而不会过度降低结合亲和力或特异性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号