当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regiodivergent Isosorbide Acylation by Oxidative N-Heterocyclic Carbene Catalysis in Batch and Continuous Flow
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-06-08 , DOI: 10.1021/acssuschemeng.1c02765 Daniele Ragno 1 , Costanza Leonardi 1 , Graziano Di Carmine 1 , Olga Bortolini 1 , Arianna Brandolese 1 , Carmela De Risi 1 , Alessandro Massi 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-06-08 , DOI: 10.1021/acssuschemeng.1c02765 Daniele Ragno 1 , Costanza Leonardi 1 , Graziano Di Carmine 1 , Olga Bortolini 1 , Arianna Brandolese 1 , Carmela De Risi 1 , Alessandro Massi 1
Affiliation
|
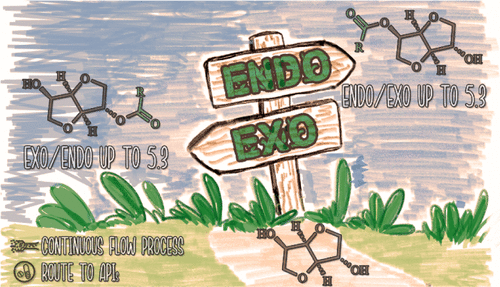
A new N-heterocyclic carbene (NHC)-catalyzed strategy for the regioselective monoesterification of isosorbide (IS) at either the endo (5-OH) or exo (2-OH) position is described. Site-selective acylation proceeds under oxidative conditions in the presence of a quinone oxidant using aldehydes as mild acylating agents. Experimental evidences suggest a role of the stereoelectronic features of the acyl azolium salt intermediate in determining the selectivity of the acylation process. The solvent effect was also investigated, considering conventional and sustainable solvents. Aromatic aldehydes, including bio-based furfural and 5-hydroxymethyl furfural, together with α,β-unsaturated aldehydes proved to be effective reaction partners affording monoacyl-isosorbides with satisfactory levels of regioselectivity (exo/endo: 5.3–3.5; endo/exo: 5.3–3.3). Additionally, the exo-selective triazolium salt promoter was successfully transferred into the heterogeneous phase and applied to continuous-flow catalysis. In particular, the polystyrene-supported version of the selected NHC showed a catalytic activity comparable to that of the homogeneous counterpart in terms of both conversion efficiency (turnover number = 108) and regioselectivity (exo/endo up to 5.3). Also, the corresponding packed-bed mesoreactor was operated with long-term stability (ca. 110 h on stream) to produce 2-benzoyl-IS (1.32 mmol h–1 mmolcat–1), which is the key intermediate in the synthesis of a commercial active pharmaceutical ingredient, namely, the vasodilator isosorbide-5-mononitrate.
中文翻译:

氧化N-杂环卡宾分批和连续流催化区域发散异山梨醇酰化
描述了一种新的 N-杂环卡宾 (NHC) 催化策略,用于在内部 (5-OH) 或外部 (2-OH) 位置对异山梨醇 (IS) 进行区域选择性单酯化。位点选择性酰化在氧化条件下在醌氧化剂存在下进行,使用醛作为温和的酰化剂。实验证据表明酰基唑鎓盐中间体的立体电子特征在决定酰化过程的选择性中的作用。考虑到常规和可持续溶剂,还研究了溶剂效应。芳香醛,包括生物基糠醛和 5-羟甲基糠醛,与 α,β-不饱和醛一起被证明是有效的反应伙伴,提供具有令人满意的区域选择性水平的单酰基异山梨醇(外/内:5.3-3.5;内/外: 5.3–3.3)。此外,外选择性三唑鎓盐促进剂成功转移到非均相中并应用于连续流催化。特别是,所选 NHC 的聚苯乙烯负载版本在转化效率(周转数 = 108)和区域选择性(exo/endo 高达 5.3)方面显示出与均相对应物相当的催化活性。此外,相应的填充床中观反应器以长期稳定性(约 110 小时运行)运行,以生产 2-苯甲酰-IS(1.32 mmol h 在转化效率(周转数 = 108)和区域选择性(exo/endo 高达 5.3)方面,所选 NHC 的聚苯乙烯负载版本显示出与均相对应物相当的催化活性。此外,相应的填充床中观反应器以长期稳定性(约 110 小时运行)运行以生产 2-苯甲酰-IS(1.32 mmol h 在转化效率(周转数 = 108)和区域选择性(exo/endo 高达 5.3)方面,所选 NHC 的聚苯乙烯负载版本显示出与均相对应物相当的催化活性。此外,相应的填充床中观反应器以长期稳定性(约 110 小时运行)运行,以生产 2-苯甲酰-IS(1.32 mmol h–1 mmol cat –1 ),这是合成商业活性药物成分的关键中间体,即血管扩张剂异山梨醇-5-单硝酸酯。
更新日期:2021-06-21
中文翻译:

氧化N-杂环卡宾分批和连续流催化区域发散异山梨醇酰化
描述了一种新的 N-杂环卡宾 (NHC) 催化策略,用于在内部 (5-OH) 或外部 (2-OH) 位置对异山梨醇 (IS) 进行区域选择性单酯化。位点选择性酰化在氧化条件下在醌氧化剂存在下进行,使用醛作为温和的酰化剂。实验证据表明酰基唑鎓盐中间体的立体电子特征在决定酰化过程的选择性中的作用。考虑到常规和可持续溶剂,还研究了溶剂效应。芳香醛,包括生物基糠醛和 5-羟甲基糠醛,与 α,β-不饱和醛一起被证明是有效的反应伙伴,提供具有令人满意的区域选择性水平的单酰基异山梨醇(外/内:5.3-3.5;内/外: 5.3–3.3)。此外,外选择性三唑鎓盐促进剂成功转移到非均相中并应用于连续流催化。特别是,所选 NHC 的聚苯乙烯负载版本在转化效率(周转数 = 108)和区域选择性(exo/endo 高达 5.3)方面显示出与均相对应物相当的催化活性。此外,相应的填充床中观反应器以长期稳定性(约 110 小时运行)运行,以生产 2-苯甲酰-IS(1.32 mmol h 在转化效率(周转数 = 108)和区域选择性(exo/endo 高达 5.3)方面,所选 NHC 的聚苯乙烯负载版本显示出与均相对应物相当的催化活性。此外,相应的填充床中观反应器以长期稳定性(约 110 小时运行)运行以生产 2-苯甲酰-IS(1.32 mmol h 在转化效率(周转数 = 108)和区域选择性(exo/endo 高达 5.3)方面,所选 NHC 的聚苯乙烯负载版本显示出与均相对应物相当的催化活性。此外,相应的填充床中观反应器以长期稳定性(约 110 小时运行)运行,以生产 2-苯甲酰-IS(1.32 mmol h–1 mmol cat –1 ),这是合成商业活性药物成分的关键中间体,即血管扩张剂异山梨醇-5-单硝酸酯。










































 京公网安备 11010802027423号
京公网安备 11010802027423号