当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of [2.2]Paracyclophane-Based Glycidic Amides Using Chiral Ammonium Ylides
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-06-07 , DOI: 10.1002/hlca.202100073 David Weinzierl 1 , Mario Waser 2
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-06-07 , DOI: 10.1002/hlca.202100073 David Weinzierl 1 , Mario Waser 2
Affiliation
|
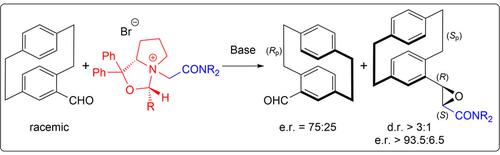
An ammonium ylide-mediated stereoselective protocol for the synthesis of a series of novel [2.2]paracyclophane-based epoxides starting from racemic 4-formyl[2.2]paracyclophane has been developed. By using achiral ammonium salts as ylide precursors, the corresponding epoxide products were obtained in isolated yields up to 76 % and with diastereoselectivities up to d.r.=9 : 1. When carrying out the reaction with chiral ammonium salts instead, the products were accessible with e.r.>93.5 : 6.5 and d.r.>3 : 1, accompanied with a moderately enantioselective kinetic resolution of the racemic starting aldehyde (e.r.=75 : 25).
中文翻译:

使用手性叶立德铵合成 [2.2] 对环芳基缩水甘油酰胺
已经开发了一种用于从外消旋 4-甲酰基 [2.2] 对环烷开始合成一系列新型 [2.2] 对环芳基环氧化物的铵叶立德介导的立体选择性方案。通过使用非手性铵盐作为叶立德前体,以高达 76 % 的分离产率和高达 9 : 1 的非对映选择性获得了相应的环氧化物产物。 > 93.5 : 6.5 和 dr > 3 : 1,伴随着外消旋起始醛的适度对映选择性动力学拆分(er = 75 : 25)。
更新日期:2021-07-16
中文翻译:

使用手性叶立德铵合成 [2.2] 对环芳基缩水甘油酰胺
已经开发了一种用于从外消旋 4-甲酰基 [2.2] 对环烷开始合成一系列新型 [2.2] 对环芳基环氧化物的铵叶立德介导的立体选择性方案。通过使用非手性铵盐作为叶立德前体,以高达 76 % 的分离产率和高达 9 : 1 的非对映选择性获得了相应的环氧化物产物。 > 93.5 : 6.5 和 dr > 3 : 1,伴随着外消旋起始醛的适度对映选择性动力学拆分(er = 75 : 25)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号