当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and Theoretical Study of CO2 Solubility in Liquid CH4/H2 Mixtures at Cryogenic Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-08 , DOI: 10.1021/acs.jced.1c00223 Jingxuan Xu 1 , Ting He 1 , Wensheng Lin 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-08 , DOI: 10.1021/acs.jced.1c00223 Jingxuan Xu 1 , Ting He 1 , Wensheng Lin 1
Affiliation
|
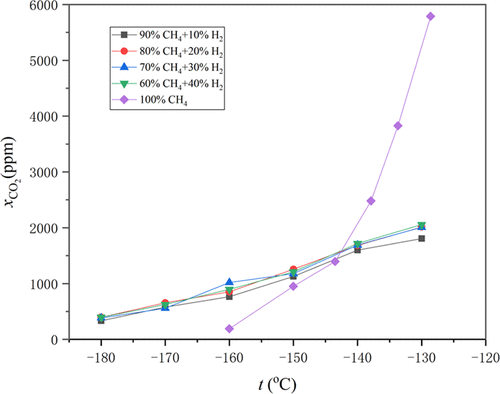
Methane-rich industrial byproducts, such as coke oven gas (COG) and synthetic ammonia exhaust tail gas, are often regarded as promising sources of unconventional natural gas. The design spec for liquefaction of these kinds of unconventional natural gas is quite different from that of conventional natural gas due to the presence of some special substances, such as hydrogen. In this work, CO2 solubility in liquid CH4/H2 mixtures is measured utilizing a static solid–liquid equilibrium (SLE) experimental apparatus. The experiments are carried out at cryogenic temperatures between 90 and 150 K in the pressure range of about 1.7–5.4 MPa. The results are compared with calculations based on the GERG-2008 and Peng–Robinson (PR) equations of state. It is found that, compared with the solubility of CO2 in pure methane, the existence of hydrogen makes CO2 solubility increase significantly between −160 and −145 °C and decrease significantly at temperatures above −140 °C. The results provide the basis for the CO2 purification system of the coke oven gas and synthetic natural gas liquefaction plant.
中文翻译:

低温下液态CH 4 /H 2混合物中CO 2溶解度的实验和理论研究
富含甲烷的工业副产品,如焦炉煤气(COG)和合成氨尾气,通常被认为是非常规天然气的有前途的来源。这类非常规天然气液化的设计规范与常规天然气的液化设计规范有很大不同,这是由于存在一些特殊物质,如氢气。在这项工作中,CO 2在液态 CH 4 /H 2 中的溶解度使用静态固液平衡 (SLE) 实验装置测量混合物。实验是在 90 到 150 K 之间的低温和大约 1.7-5.4 MPa 的压力范围内进行的。将结果与基于 GERG-2008 和 Peng-Robinson (PR) 状态方程的计算进行比较。研究发现,与CO 2在纯甲烷中的溶解度相比,氢气的存在使得CO 2 的溶解度在-160~-145℃之间显着增加,在-140℃以上时显着降低。研究结果为焦炉煤气和合成天然气液化装置的CO 2净化系统提供了依据。
更新日期:2021-07-08
中文翻译:

低温下液态CH 4 /H 2混合物中CO 2溶解度的实验和理论研究
富含甲烷的工业副产品,如焦炉煤气(COG)和合成氨尾气,通常被认为是非常规天然气的有前途的来源。这类非常规天然气液化的设计规范与常规天然气的液化设计规范有很大不同,这是由于存在一些特殊物质,如氢气。在这项工作中,CO 2在液态 CH 4 /H 2 中的溶解度使用静态固液平衡 (SLE) 实验装置测量混合物。实验是在 90 到 150 K 之间的低温和大约 1.7-5.4 MPa 的压力范围内进行的。将结果与基于 GERG-2008 和 Peng-Robinson (PR) 状态方程的计算进行比较。研究发现,与CO 2在纯甲烷中的溶解度相比,氢气的存在使得CO 2 的溶解度在-160~-145℃之间显着增加,在-140℃以上时显着降低。研究结果为焦炉煤气和合成天然气液化装置的CO 2净化系统提供了依据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号