当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Use of Cause-and-Effect Analysis to Optimize the Reliability of In Vitro Inhalation Toxicity Measurements Using an Air–Liquid Interface
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2021-06-07 , DOI: 10.1021/acs.chemrestox.1c00080 Elijah J Petersen 1 , Monita Sharma 2 , Amy J Clippinger 2 , John Gordon 3 , Aaron Katz 4 , Peter Laux 4 , Lars B Leibrock 4 , Andreas Luch 4 , Joanna Matheson 3 , Andreas O Stucki 2 , Jutta Tentschert 4 , Frank S Bierkandt 4
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2021-06-07 , DOI: 10.1021/acs.chemrestox.1c00080 Elijah J Petersen 1 , Monita Sharma 2 , Amy J Clippinger 2 , John Gordon 3 , Aaron Katz 4 , Peter Laux 4 , Lars B Leibrock 4 , Andreas Luch 4 , Joanna Matheson 3 , Andreas O Stucki 2 , Jutta Tentschert 4 , Frank S Bierkandt 4
Affiliation
|
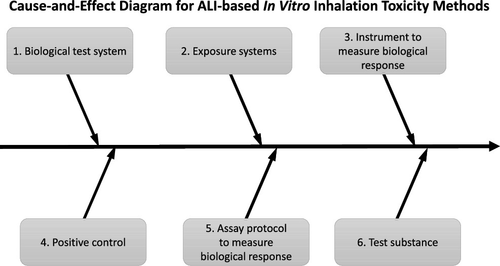
In vitro inhalation toxicology methods are increasingly being used for research and regulatory purposes. Although the opportunity for increased human relevance of in vitro inhalation methods compared to in vivo tests has been established and discussed, how to systematically account for variability and maximize the reliability of these in vitro methods, especially for assays that use cells cultured at an air–liquid interface (ALI), has received less attention. One tool that has been used to evaluate the robustness of in vitro test methods is cause-and-effect (C&E) analysis, a conceptual approach to analyze key sources of potential variability in a test method. These sources of variability can then be evaluated using robustness testing and potentially incorporated into in-process control measurements in the assay protocol. There are many differences among in vitro inhalation test methods including the use of different types of biological test systems, exposure platforms/conditions, substances tested, and end points, which represent a major challenge for use in regulatory testing. In this manuscript, we describe how C&E analysis can be applied using a modular approach based on the idea that shared components of different test methods (e.g., the same exposure system is used) have similar sources of variability even though other components may differ. C&E analyses of different in vitro inhalation methods revealed a common set of recommended exposure systems and biological in-process control measurements. The approach described here, when applied in conjunction with Good Laboratory Practices (GLP) criteria, should help improve the inter- and intralaboratory agreement of in vitro inhalation test results, leading to increased confidence in these methods for regulatory and research purposes.
中文翻译:

使用因果分析来优化使用气液界面的体外吸入毒性测量的可靠性
体外吸入毒理学方法越来越多地用于研究和监管目的。尽管已经建立并讨论了与体内试验相比体外吸入方法增加人类相关性的机会,但如何系统地考虑变异性并最大限度地提高这些体外方法的可靠性,尤其是对于使用在空气中培养的细胞的分析——液体界面(ALI),很少受到关注。一种用于评估体外稳健性的工具测试方法是因果 (C&E) 分析,这是一种分析测试方法中潜在变异性关键来源的概念方法。然后可以使用稳健性测试评估这些可变性来源,并可能将其纳入分析协议中的过程控制测量中。体外有很多差异吸入测试方法包括使用不同类型的生物测试系统、暴露平台/条件、测试的物质和终点,这些都是监管测试中使用的主要挑战。在这份手稿中,我们描述了如何使用模块化方法应用 C&E 分析,该方法基于不同测试方法的共享组件(例如,使用相同的暴露系统)具有相似的可变性来源,即使其他组件可能不同。对不同体外吸入方法的C&E 分析揭示了一组通用的推荐暴露系统和生物过程控制测量。此处描述的方法与良好实验室规范 (GLP) 标准结合使用时,应有助于改进实验室间和实验室内的一致性体外吸入测试结果,增加了对这些方法用于监管和研究目的的信心。
更新日期:2021-06-21
中文翻译:

使用因果分析来优化使用气液界面的体外吸入毒性测量的可靠性
体外吸入毒理学方法越来越多地用于研究和监管目的。尽管已经建立并讨论了与体内试验相比体外吸入方法增加人类相关性的机会,但如何系统地考虑变异性并最大限度地提高这些体外方法的可靠性,尤其是对于使用在空气中培养的细胞的分析——液体界面(ALI),很少受到关注。一种用于评估体外稳健性的工具测试方法是因果 (C&E) 分析,这是一种分析测试方法中潜在变异性关键来源的概念方法。然后可以使用稳健性测试评估这些可变性来源,并可能将其纳入分析协议中的过程控制测量中。体外有很多差异吸入测试方法包括使用不同类型的生物测试系统、暴露平台/条件、测试的物质和终点,这些都是监管测试中使用的主要挑战。在这份手稿中,我们描述了如何使用模块化方法应用 C&E 分析,该方法基于不同测试方法的共享组件(例如,使用相同的暴露系统)具有相似的可变性来源,即使其他组件可能不同。对不同体外吸入方法的C&E 分析揭示了一组通用的推荐暴露系统和生物过程控制测量。此处描述的方法与良好实验室规范 (GLP) 标准结合使用时,应有助于改进实验室间和实验室内的一致性体外吸入测试结果,增加了对这些方法用于监管和研究目的的信心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号