当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Temperature dependence of the gas-particle partitioning of selected VOCs
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2021-6-3 , DOI: 10.1039/d1em00176k Jeonghyeon Ahn 1 , Guiying Rao 1 , Eric Vejerano 1
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2021-6-3 , DOI: 10.1039/d1em00176k Jeonghyeon Ahn 1 , Guiying Rao 1 , Eric Vejerano 1
Affiliation
|
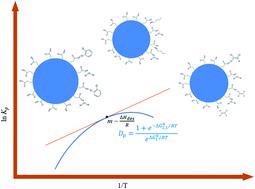
The gas-particle partitioning coefficients for volatile organic compounds (VOCs) are difficult to acquire because discriminating the small mass fraction of the VOCs in the aerosol particle relative to that in the gas phase is challenging. In this paper, we report the temperature dependence of the gas-particle partitioning coefficient (Kp) for n-butanol (n-BuOH) and trichloroethylene (TCE). Using the bench-scale system that we developed, we measured the Kp of surrogate VOCs, n-BuOH, and TCE onto inorganic (ammonium sulfate, Am Sulf) and organic (succinic acid, SA) aerosol particles at a fixed relative humidity (RH) of 35%. At this RH level and temperature range of 278.15–308.15 K, the ln Kp for TCE and n-BuOH partitioning on SA aerosol particles were −27.0 ± 0.70 to −27.9 ± 0.01 and −13.9 ± 0.03 to −17.4 ± 0.17. In contrast, the ln Kp for TCE and n-BuOH partitioning on Am Sulf aerosol particles ranged from −26.4 ± 0.70 to −27.4 ± 0.71 and −14.1 ± 0.03 to −17.1 ± 0.17, respectively. Results showed that TCE fitted well with the classic van't Hoff relationship. The enthalpy of desorption (ΔHdes) for TCE was constant over the temperature range of 278.15 K to 308.15 K, behaving similarly to 1,2-dichlorobenzene. At a similar temperature range, n-BuOH partitioning into both aerosol particles exhibited nonlinear temperature dependence. The minimum ratio of ΔHdes (Am Sulf:SA) for n-BuOH partitioning on each aerosol type was at ∼278.15 K. The magnitude of the entropy ΔSdes for all VOCs was <1 kJ mol−1.
中文翻译:

选定 VOC 的气体-颗粒分配的温度依赖性
挥发性有机化合物 (VOC) 的气体-颗粒分配系数很难获得,因为区分气溶胶颗粒中相对于气相中的 VOC 的小质量分数具有挑战性。在本文中,我们报告了正丁醇 ( n -BuOH) 和三氯乙烯 (TCE)的气体-颗粒分配系数 ( K p )的温度依赖性。使用我们开发的实验室规模系统,我们测量了替代 VOC的K p,n-BuOH 和 TCE 在 35% 的固定相对湿度 (RH) 下附着在无机(硫酸铵,Am Sulf)和有机(琥珀酸,SA)气溶胶颗粒上。在 RH 水平和 278.15–308.15 K 的温度范围内,TCE 和n -BuOH 在 SA 气溶胶颗粒上分配的 ln K p为-27.0 ± 0.70 至 -27.9 ± 0.01 和 -13.9 ± 0.03 至 -17.4 ± 0.17。相比之下,TCE 和n -BuOH 在 Am Sulf 气溶胶颗粒上分配的 ln K p范围分别为 -26.4 ± 0.70 至 -27.4 ± 0.71 和 -14.1 ± 0.03 至 -17.1 ± 0.17。结果表明,TCE 与经典的 van't Hoff 关系非常吻合。解吸焓 (Δ H des) 对于 TCE,在 278.15 K 到 308.15 K 的温度范围内是恒定的,其行为类似于 1,2-二氯苯。在相似的温度范围内,n- BuOH 分配到两种气溶胶颗粒中表现出非线性温度依赖性。每种气溶胶类型上n -BuOH 分配的 Δ H des (Am Sulf:SA)的最小比率为 ~ 278.15 K。所有 VOC的熵 Δ S des的大小小于 1 kJ mol -1。
更新日期:2021-06-08
中文翻译:

选定 VOC 的气体-颗粒分配的温度依赖性
挥发性有机化合物 (VOC) 的气体-颗粒分配系数很难获得,因为区分气溶胶颗粒中相对于气相中的 VOC 的小质量分数具有挑战性。在本文中,我们报告了正丁醇 ( n -BuOH) 和三氯乙烯 (TCE)的气体-颗粒分配系数 ( K p )的温度依赖性。使用我们开发的实验室规模系统,我们测量了替代 VOC的K p,n-BuOH 和 TCE 在 35% 的固定相对湿度 (RH) 下附着在无机(硫酸铵,Am Sulf)和有机(琥珀酸,SA)气溶胶颗粒上。在 RH 水平和 278.15–308.15 K 的温度范围内,TCE 和n -BuOH 在 SA 气溶胶颗粒上分配的 ln K p为-27.0 ± 0.70 至 -27.9 ± 0.01 和 -13.9 ± 0.03 至 -17.4 ± 0.17。相比之下,TCE 和n -BuOH 在 Am Sulf 气溶胶颗粒上分配的 ln K p范围分别为 -26.4 ± 0.70 至 -27.4 ± 0.71 和 -14.1 ± 0.03 至 -17.1 ± 0.17。结果表明,TCE 与经典的 van't Hoff 关系非常吻合。解吸焓 (Δ H des) 对于 TCE,在 278.15 K 到 308.15 K 的温度范围内是恒定的,其行为类似于 1,2-二氯苯。在相似的温度范围内,n- BuOH 分配到两种气溶胶颗粒中表现出非线性温度依赖性。每种气溶胶类型上n -BuOH 分配的 Δ H des (Am Sulf:SA)的最小比率为 ~ 278.15 K。所有 VOC的熵 Δ S des的大小小于 1 kJ mol -1。









































 京公网安备 11010802027423号
京公网安备 11010802027423号