当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asp56 in actin is critical for the full activity of the amino acid starvation-responsive kinase Gcn2
FEBS Letters ( IF 3.5 ) Pub Date : 2021-06-06 , DOI: 10.1002/1873-3468.14137 Rashmi Ramesh 1 , Martina Dautel 1 , Yongook Lee 2 , Yeonsoo Kim 2 , Kirsty Storey 1 , Susanne Gottfried 1 , Terri Goss Kinzy 3 , Won-Ki Huh 2 , Evelyn Sattlegger 1, 4, 5
FEBS Letters ( IF 3.5 ) Pub Date : 2021-06-06 , DOI: 10.1002/1873-3468.14137 Rashmi Ramesh 1 , Martina Dautel 1 , Yongook Lee 2 , Yeonsoo Kim 2 , Kirsty Storey 1 , Susanne Gottfried 1 , Terri Goss Kinzy 3 , Won-Ki Huh 2 , Evelyn Sattlegger 1, 4, 5
Affiliation
|
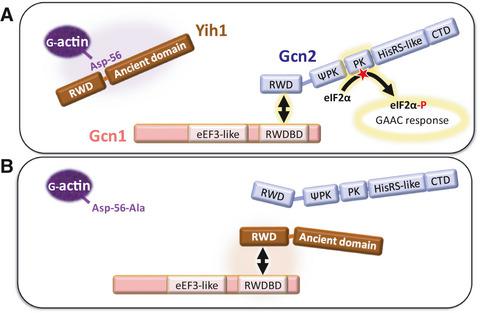
Eukaryotes harbour a conserved signalling pathway, called General Amino Acid Control (GAAC) in Saccharomyces cerevisiae, for overcoming amino acid starvation. Upon starvation, the protein kinase Gcn2, which phosphorylates the eukaryotic translation initiation factor eIF2α, becomes stimulated to trigger the GAAC response. Genetic studies suggest that Yih1, which is the yeast homolog of mammalian IMPACT and which binds monomeric actin, inhibits Gcn2 when released from actin. Here, we found that D56A substitution in actin (the act1-9 allele) leads to reduced eIF2α phosphorylation, suggesting that the Asp56 residue is required for full Gcn2 activation. In the act1-9 mutant, Yih1 overexpression further enhanced the sensitivity to amino acid starvation-inducing drugs and further impaired eIF2α phosphorylation, suggesting that Gcn2 inhibition was mediated via Yih1. The D56A substitution may impair the actin–Yih1 interaction, directly or indirectly, thereby increasing the amount of Yih1 available to inhibit Gcn2.
中文翻译:

肌动蛋白中的 Asp56 对氨基酸饥饿反应激酶 Gcn2 的全部活性至关重要
真核生物具有保守的信号通路,称为酿酒酵母中的一般氨基酸控制 (GAAC) ,用于克服氨基酸饥饿。饥饿时,使真核翻译起始因子 eIF2α 磷酸化的蛋白激酶 Gcn2 被刺激以触发 GAAC 反应。遗传研究表明,Yih1 是哺乳动物 IMPACT 的酵母同源物并结合单体肌动蛋白,当从肌动蛋白释放时会抑制 Gcn2。在这里,我们发现肌动蛋白(act1-9等位基因)中的 D56A 取代导致 eIF2α 磷酸化降低,表明 Asp56 残基是完全 Gcn2 激活所必需的。在行动 1-9在突变体中,Yih1 过表达进一步增强了对氨基酸饥饿诱导药物的敏感性并进一步削弱了 eIF2α 磷酸化,表明 Gcn2 抑制是通过Yih1介导的。D56A 取代可能会直接或间接损害肌动蛋白-Yih1 的相互作用,从而增加可用于抑制 Gcn2 的 Yih1 的量。
更新日期:2021-07-27
中文翻译:

肌动蛋白中的 Asp56 对氨基酸饥饿反应激酶 Gcn2 的全部活性至关重要
真核生物具有保守的信号通路,称为酿酒酵母中的一般氨基酸控制 (GAAC) ,用于克服氨基酸饥饿。饥饿时,使真核翻译起始因子 eIF2α 磷酸化的蛋白激酶 Gcn2 被刺激以触发 GAAC 反应。遗传研究表明,Yih1 是哺乳动物 IMPACT 的酵母同源物并结合单体肌动蛋白,当从肌动蛋白释放时会抑制 Gcn2。在这里,我们发现肌动蛋白(act1-9等位基因)中的 D56A 取代导致 eIF2α 磷酸化降低,表明 Asp56 残基是完全 Gcn2 激活所必需的。在行动 1-9在突变体中,Yih1 过表达进一步增强了对氨基酸饥饿诱导药物的敏感性并进一步削弱了 eIF2α 磷酸化,表明 Gcn2 抑制是通过Yih1介导的。D56A 取代可能会直接或间接损害肌动蛋白-Yih1 的相互作用,从而增加可用于抑制 Gcn2 的 Yih1 的量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号