当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Behavior of N-Carbobenzoxy-l-2-phenylglycine in 11 Pure and a Binary Ethanol + Water Solvent Systems at 283.15–323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-06 , DOI: 10.1021/acs.jced.1c00240 Yue Zhao 1, 2 , Jingxuan Qiu 3 , Shen Hu 2, 3 , Haishuang Huang 3 , Hui He 3 , Ying Guo 2, 3 , Haoyou Liu 2, 3 , Jiaming Han 2, 3 , Peng Wang 2, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-06-06 , DOI: 10.1021/acs.jced.1c00240 Yue Zhao 1, 2 , Jingxuan Qiu 3 , Shen Hu 2, 3 , Haishuang Huang 3 , Hui He 3 , Ying Guo 2, 3 , Haoyou Liu 2, 3 , Jiaming Han 2, 3 , Peng Wang 2, 3
Affiliation
|
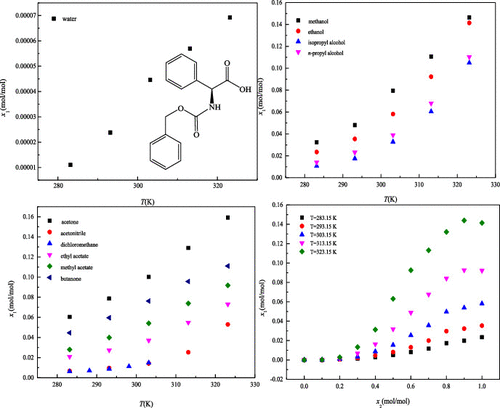
The mole fraction solubility data of N-carboxyphenoxy-L-2-phenylglycine in 11 neat solvents (methanol, water, isopropyl alcohol, ethanol, n-propyl alcohol, methyl acetate, ethyl acetate, acetone, dichloromethane, acetonitrile, and butanone) and a binary solvent mixture of ethanol + water were determined by the static gravimetric method at 283.15–323.15 K. From the solvent effect analysis, the solubility behavior was affected not only by polarity, but also by some other factors such as hydrogen bonding, cohesive energy density, and molecular structure. The solubility data of N-carboxyphenoxy-L-2-phenylglycine were correlated by using the two-dimensional modified Apelblat model and three-dimensional Apelblat–Jouyban–Acree model. The maximum values of root-mean-square deviation and relative average deviation were 100.98 × 10–4 and 20.53%, respectively. The solubility calculated by the two models agreed well with the experimental values.
中文翻译:

N-羧基苯甲氧基-1-2-苯基甘氨酸在 11 纯和二元乙醇 + 水溶剂系统中的溶解度行为,温度为 283.15–323.15 K
的摩尔分数溶解度数据Ñ -carboxyphenoxy-大号在11种整齐溶剂(甲醇,水,异丙醇,乙醇,-2-苯基甘氨酸Ñ丙基醇,乙酸甲酯,乙酸乙酯,丙酮,二氯甲烷,乙腈,和丁酮),并乙醇+水的二元溶剂混合物在283.15-323.15 K下通过静态重量法测定。从溶剂效应分析,溶解行为不仅受极性影响,还受氢键、内聚能等其他因素的影响密度和分子结构。N-羧基苯氧基-L的溶解度数据-2-苯基甘氨酸通过使用二维修正的 Apelblat 模型和三维 Apelblat-Jouyban-Acree 模型进行关联。均方根偏差和相对平均偏差的最大值分别为 100.98 × 10 –4和 20.53%。两种模型计算的溶解度与实验值吻合较好。
更新日期:2021-07-08
中文翻译:

N-羧基苯甲氧基-1-2-苯基甘氨酸在 11 纯和二元乙醇 + 水溶剂系统中的溶解度行为,温度为 283.15–323.15 K
的摩尔分数溶解度数据Ñ -carboxyphenoxy-大号在11种整齐溶剂(甲醇,水,异丙醇,乙醇,-2-苯基甘氨酸Ñ丙基醇,乙酸甲酯,乙酸乙酯,丙酮,二氯甲烷,乙腈,和丁酮),并乙醇+水的二元溶剂混合物在283.15-323.15 K下通过静态重量法测定。从溶剂效应分析,溶解行为不仅受极性影响,还受氢键、内聚能等其他因素的影响密度和分子结构。N-羧基苯氧基-L的溶解度数据-2-苯基甘氨酸通过使用二维修正的 Apelblat 模型和三维 Apelblat-Jouyban-Acree 模型进行关联。均方根偏差和相对平均偏差的最大值分别为 100.98 × 10 –4和 20.53%。两种模型计算的溶解度与实验值吻合较好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号