当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fractionation of Oxygen Isotopes in Uranium Oxides during Peroxide Precipitation and Dry Air Calcination
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-06-04 , DOI: 10.1021/acsearthspacechem.1c00112 Michael R. Klosterman 1 , Erik J. Oerter 2 , Suvankar Chakraborty 3 , Michael J. Singleton 2 , Luther W. McDonald 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-06-04 , DOI: 10.1021/acsearthspacechem.1c00112 Michael R. Klosterman 1 , Erik J. Oerter 2 , Suvankar Chakraborty 3 , Michael J. Singleton 2 , Luther W. McDonald 1
Affiliation
|
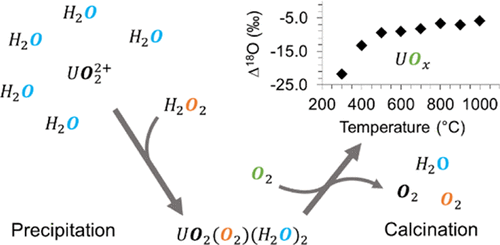
The interaction between uranium and oxygen-containing substrates is nearly ubiquitous within the nuclear fuel cycle. Given the well-known and predictable oxygen stable isotope compositions of atmospheric oxygen and meteoric waters around the world, the use of these isotopes as a potential geolocation or processing signature of uranium compounds has been of interest within the nuclear safeguards community. This study focuses on measuring the oxygen-stable isotope composition of synthetically produced uranium oxides to determine the mechanism and extent of fractionation during materials processing relevant to the nuclear fuel cycle. Metastudtite [UO2O2(H2O)2] samples were first produced using water and hydrogen peroxide of a known oxygen isotope composition. This starting material was calcined in dry air at temperatures ranging from 300 °C to 1000 °C for 20 h producing oxides ranging in composition from UO3+x (0 ≤ x ≤ 0.5) to U3O8. In addition, calcinations between 500 and 800 °C were performed at different time intervals to evaluate the rate of isotopic equilibration. The δ18O values of water bound to metastudtite were measured by thermogravimetric analysis coupled to isotope ratio infrared spectroscopy (TGA–IRIS). A redesigned fluorination manifold was constructed at the University of Utah and was used to extract the bulk oxygen from precipitated and calcined products using bromine pentafluoride (BrF5). Mineralization water on metastudtite (measured by TGA–IRIS) was closely associated with the aqueous environment of precipitation with a fractionation of +3.5 ± 0.6‰. In contrast, bulk oxygen from metastudtite (measured by fluorination and gas chromatography combined with isotope ratio mass spectrometry) was found to have an inconsistent fractionation with precipitation water. Samples calcined in dry air exhibited a wide range of oxygen isotope compositions which increased from δ18O = +1.4 ± 0.9‰ at 300 °C to +17.3 ± 0.9‰ at 1000 °C. This variation in δ18O values was a result of oxygen exchange with atmospheric O2 and was found to be rapid at calcination temperatures greater than 600°C—with equilibration occurring in less than 2 h. These results provide insights into the incorporation and exchange of oxygen isotopes during precipitation and calcination processes comparable to those employed within the nuclear fuel cycle and establish a foundation for future investigations to build upon.
中文翻译:

过氧化物沉淀和干燥空气煅烧过程中氧化铀中氧同位素的分馏
铀和含氧底物之间的相互作用在核燃料循环中几乎无处不在。鉴于世界各地大气氧和大气水域众所周知且可预测的氧稳定同位素组成,将这些同位素用作铀化合物的潜在地理定位或加工特征一直是核保障界的兴趣所在。这项研究的重点是测量合成生产的氧化铀的氧稳定同位素组成,以确定与核燃料循环相关的材料加工过程中的分馏机制和程度。Metastudtite [UO 2 O 2 (H 2 O) 2] 样品首先使用水和已知氧同位素组成的过氧化氢生产。该起始材料在干燥空气中在 300°C 至 1000°C 的温度范围内煅烧 20 小时,产生组成范围为 UO 3 + x (0≤ x ≤ 0.5) 至 U 3 O 8 的氧化物。此外,以不同的时间间隔在 500 到 800 °C 之间进行煅烧,以评估同位素平衡的速率。δ 18通过与同位素比红外光谱 (TGA-IRIS) 耦合的热重分析测量结合到偏铁矿的水的 O 值。犹他大学建造了一个重新设计的氟化歧管,用于从使用五氟化溴 (BrF 5 ) 的沉淀和煅烧产品中提取大量氧气。metastudtite 上的矿化水(通过 TGA-IRIS 测量)与分馏为 +3.5 ± 0.6‰ 的沉淀水环境密切相关。相比之下,发现来自 metastudtite 的大量氧气(通过氟化和气相色谱结合同位素比质谱法测量)与沉淀水的分馏不一致。在干燥空气中煅烧的样品表现出广泛的氧同位素组成,从 δ18 O = +1.4 ± 0.9‰ 在 300 °C 到 +17.3 ± 0.9‰ 在 1000 °C。δ 18 O 值的这种变化是氧与大气 O 2交换的结果,并且发现在高于 600°C 的煅烧温度下变化很快——平衡发生在不到 2 小时。这些结果为沉淀和煅烧过程中氧同位素的掺入和交换提供了见解,与核燃料循环中使用的那些过程相当,并为未来的研究奠定了基础。
更新日期:2021-06-17
中文翻译:

过氧化物沉淀和干燥空气煅烧过程中氧化铀中氧同位素的分馏
铀和含氧底物之间的相互作用在核燃料循环中几乎无处不在。鉴于世界各地大气氧和大气水域众所周知且可预测的氧稳定同位素组成,将这些同位素用作铀化合物的潜在地理定位或加工特征一直是核保障界的兴趣所在。这项研究的重点是测量合成生产的氧化铀的氧稳定同位素组成,以确定与核燃料循环相关的材料加工过程中的分馏机制和程度。Metastudtite [UO 2 O 2 (H 2 O) 2] 样品首先使用水和已知氧同位素组成的过氧化氢生产。该起始材料在干燥空气中在 300°C 至 1000°C 的温度范围内煅烧 20 小时,产生组成范围为 UO 3 + x (0≤ x ≤ 0.5) 至 U 3 O 8 的氧化物。此外,以不同的时间间隔在 500 到 800 °C 之间进行煅烧,以评估同位素平衡的速率。δ 18通过与同位素比红外光谱 (TGA-IRIS) 耦合的热重分析测量结合到偏铁矿的水的 O 值。犹他大学建造了一个重新设计的氟化歧管,用于从使用五氟化溴 (BrF 5 ) 的沉淀和煅烧产品中提取大量氧气。metastudtite 上的矿化水(通过 TGA-IRIS 测量)与分馏为 +3.5 ± 0.6‰ 的沉淀水环境密切相关。相比之下,发现来自 metastudtite 的大量氧气(通过氟化和气相色谱结合同位素比质谱法测量)与沉淀水的分馏不一致。在干燥空气中煅烧的样品表现出广泛的氧同位素组成,从 δ18 O = +1.4 ± 0.9‰ 在 300 °C 到 +17.3 ± 0.9‰ 在 1000 °C。δ 18 O 值的这种变化是氧与大气 O 2交换的结果,并且发现在高于 600°C 的煅烧温度下变化很快——平衡发生在不到 2 小时。这些结果为沉淀和煅烧过程中氧同位素的掺入和交换提供了见解,与核燃料循环中使用的那些过程相当,并为未来的研究奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号