当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Ferric Chloride Facilitating Efficient Lithium Extraction from Magnesium-Rich Brine with Tri-n-butyl Phosphate
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-06-03 , DOI: 10.1021/acs.iecr.1c01003 Zheng Li 1 , Koen Binnemans 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-06-03 , DOI: 10.1021/acs.iecr.1c01003 Zheng Li 1 , Koen Binnemans 1
Affiliation
|
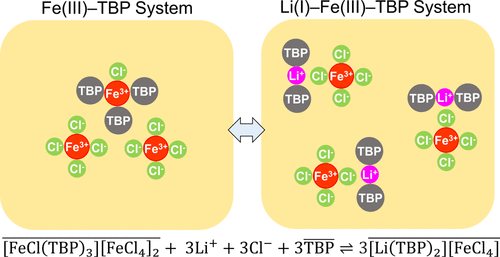
The synergistic solvent extraction system comprising tri-n-butyl phosphate (TBP) and FeCl3 has been intensively studied for selective extraction of Li(I) from Mg(II)-rich brine. The extraction occurs via the formation of an ion-pair complex [Li(TBP)x][FeCl4] in which the negatively charged [FeCl4]− neutralizes the positively charged [Li(TBP)x]+. Counterintuitively, many other metal chlorides give much lower Li(I) extraction efficiency than FeCl3, although they can also form chlorometallate anions similar to [FeCl4]−. In this study, the capabilities of CuCl2, AlCl3, InCl3, and SnCl4 for Li(I) extraction with TBP were examined and compared with that of FeCl3, accompanied by speciation studies. It was found that (1) AlCl3 does not form [AlCl4]− while other metal chlorides (CuCl2, InCl3, FeCl3, and SnCl4) can form chlorometallate anions that could facilitate Li(I) extraction and (2) CuCl2, InCl3, and SnCl4 form neutral solvation complexes with TBP strongly, which hinders the formation of chlorometallate anions, but FeCl3 does not form neutral complexes with TBP. Concurrently being able to form [FeCl4]− and to avoid forming neutral complexes facilitates the efficient Li(I) extraction by FeCl3 with TBP.
中文翻译:

氯化铁促进磷酸三正丁酯从富镁盐水中高效提锂的机理
包括三-协同溶剂萃取系统Ñ丁基磷酸酯(TBP)和的FeCl 3已被广泛研究用于从镁锂(I)的选择性提取(II)富盐水洗涤。通过形成离子对复合物 [Li(TBP) x ][FeCl 4 ] 进行萃取,其中带负电的 [FeCl 4 ] -中和带正电的 [Li(TBP) x ] +。与直觉相反,许多其他金属氯化物的 Li(I) 提取效率比 FeCl 3低得多,尽管它们也可以形成类似于 [FeCl 4 ] - 的氯金属阴离子. 在本研究中,研究了 CuCl 2、AlCl 3、InCl 3和 SnCl 4用 TBP 萃取 Li(I) 的能力,并与 FeCl 3进行了比较,同时进行了形态研究。发现 (1) AlCl 3不形成 [AlCl 4 ] -而其他金属氯化物(CuCl 2、InCl 3、FeCl 3和 SnCl 4)可以形成氯金属阴离子,可以促进 Li(I) 提取和(2 ) CuCl 2、InCl 3和 SnCl 4FeCl 3与TBP形成中性溶剂化络合物,阻碍了氯金属阴离子的形成,但FeCl 3不与TBP形成中性络合物。同时能够形成[FeCl 4 ] -并避免形成中性络合物促进了FeCl 3与TBP的有效Li(I)萃取。
更新日期:2021-06-17
中文翻译:

氯化铁促进磷酸三正丁酯从富镁盐水中高效提锂的机理
包括三-协同溶剂萃取系统Ñ丁基磷酸酯(TBP)和的FeCl 3已被广泛研究用于从镁锂(I)的选择性提取(II)富盐水洗涤。通过形成离子对复合物 [Li(TBP) x ][FeCl 4 ] 进行萃取,其中带负电的 [FeCl 4 ] -中和带正电的 [Li(TBP) x ] +。与直觉相反,许多其他金属氯化物的 Li(I) 提取效率比 FeCl 3低得多,尽管它们也可以形成类似于 [FeCl 4 ] - 的氯金属阴离子. 在本研究中,研究了 CuCl 2、AlCl 3、InCl 3和 SnCl 4用 TBP 萃取 Li(I) 的能力,并与 FeCl 3进行了比较,同时进行了形态研究。发现 (1) AlCl 3不形成 [AlCl 4 ] -而其他金属氯化物(CuCl 2、InCl 3、FeCl 3和 SnCl 4)可以形成氯金属阴离子,可以促进 Li(I) 提取和(2 ) CuCl 2、InCl 3和 SnCl 4FeCl 3与TBP形成中性溶剂化络合物,阻碍了氯金属阴离子的形成,但FeCl 3不与TBP形成中性络合物。同时能够形成[FeCl 4 ] -并避免形成中性络合物促进了FeCl 3与TBP的有效Li(I)萃取。











































 京公网安备 11010802027423号
京公网安备 11010802027423号