Journal of Materiomics ( IF 8.4 ) Pub Date : 2021-06-05 , DOI: 10.1016/j.jmat.2021.05.006 Bo Liu , Qianglin Hu , Tianyu Gao , Peiguang Liao , Yufeng Wen , Ziheng Lu , Jiong Yang , Siqi Shi , Wenqing Zhang
|
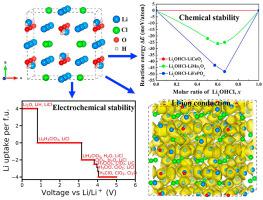
Lithium-rich antiperovskites are promising solid-state electrolytes for all-solid-state lithium-ion batteries because of their high structural tolerance and good formability. However, the experimentally reported proton-free Li3OCl is plagued by its inferior interfacial compatibility and harsh synthesis conditions. In contrast, Li2OHCl is a thermodynamically favored phases and is easier to achieve than Li3OCl. Due to the proton inside this material, it exhibits interesting lithium diffusion mechanisms. Herein, we present a systematic investigation of the ionic transport, phase stability, and electrochemical-chemical stability of Li2OHCl using first-principles calculations. Our results indicate that Li2OHCl is thermodynamically metastable and is an electronic insulator. The wide electrochemical stability window and high chemical stability of Li2OHCl against various electrodes are confirmed. The charged defects are the dominant conduction mechanism for Li-transport, with a low energy barrier of ∼0.50 eV. The Li-ion conductivity estimated by ab initio molecular dynamics simulations is about 1.3 × 10−4 S cm−1 at room temperature. This work identifies the origin of the high interfacial stability and ionic conductivity of Li2OHCl, which can further lead to the design of such as a cathode coating. Moreover, all computational methods for calculating the properties of Li2OHCl are general and can guide the design of high-performance solid-state electrolytes.
中文翻译:

对 Li2OHC 固态电解质的离子传输机制和界面稳定性的计算洞察
富锂反钙钛矿因其高结构耐受性和良好的成型性而成为全固态锂离子电池的有前途的固态电解质。然而,实验报告的无质子 Li 3 OCl 受到其较差的界面相容性和苛刻的合成条件的困扰。相比之下,Li 2 OHCl 是热力学有利的相,并且比 Li 3 OCl更容易实现。由于这种材料内部的质子,它表现出有趣的锂扩散机制。在此,我们使用第一性原理计算对 Li 2 OHCl的离子传输、相稳定性和电化学-化学稳定性进行了系统研究。我们的结果表明,Li 2OHCl 是热力学亚稳态的,是一种电子绝缘体。证实了 Li 2 OHCl 对各种电极的宽电化学稳定性窗口和高化学稳定性。带电缺陷是锂传输的主要传导机制,具有约 0.50 eV 的低能垒。通过从头分子动力学模拟估计的锂离子电导率在室温下约为 1.3 × 10 -4 S cm -1。这项工作确定了 Li 2 OHCl的高界面稳定性和离子电导率的起源,这可以进一步导致诸如阴极涂层的设计。此外,用于计算 Li 2性质的所有计算方法OHCl 是通用的,可以指导高性能固态电解质的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号