Insect Biochemistry and Molecular Biology ( IF 3.2 ) Pub Date : 2021-06-05 , DOI: 10.1016/j.ibmb.2021.103607 Valeriya Zabelina 1 , Yoko Takasu 2 , Hana Sehadova 3 , Naoyuki Yonemura 2 , Kenichi Nakajima 2 , Hideki Sezutsu 2 , Michal Sery 4 , Michal Zurovec 3 , Frantisek Sehnal 4 , Toshiki Tamura 5
|
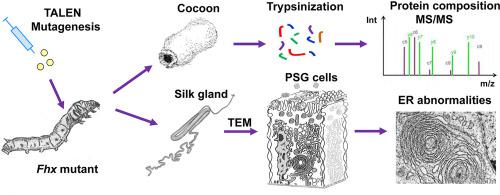
Larvae of many lepidopteran species produce a mixture of secretory proteins, known as silk, for building protective shelters and cocoons. Silk consists of a water-insoluble silk filament core produced in the posterior silk gland (PSG) and a sticky hydrophilic coating produced by the middle silk gland (MSG). In Bombyx mori, the fiber core comprises three proteins: heavy chain fibroin (Fib-H), light chain fibroin (Fib-L) and fibrohexamerin (Fhx, previously referred to as P25). To learn more about the role of Fhx, we used transcription activator-like effector nuclease (TALEN) mutagenesis and prepared a homozygous line with a null mutation in the Fhx gene. Our characterization of cocoon morphology and silk quality showed that the mutation had very little effect. However, a detailed inspection of the secretory cells in the posterior silk gland (PSG) of mid-last-instar mutant larvae revealed temporary changes in the morphology of the endoplasmic reticulum. We also observed a morphological difference in fibroin secretory globules stored in the PSG lumen of Fhx mutants, which suggests that their fibroin complexes have a slightly lower solubility. Finally, we performed an LC-MS-based quantitative proteomic analysis comparing mutant and wild-type (wt) cocoon proteins and found a high abundance of a 16 kDa secretory protein likely involved in fibroin solubility. Overall, our study shows that whilst Fhx is dispensable for silk formation, it contributes to the stability of fibroin complexes during intracellular transport and affects the morphology of fibroin secretory globules in the PSG lumen.
中文翻译:

家蚕纤维六聚体蛋白 (P25) 基因的突变导致后部丝腺细胞中粗面内质网的重组并改变丝腺腔中丝素蛋白分泌小球的形态
许多鳞翅目物种的幼虫会产生一种称为丝的分泌蛋白混合物,用于建造保护性的庇护所和茧。蚕丝由后部丝腺 (PSG) 产生的不溶于水的丝芯和中部丝腺 (MSG) 产生的粘性亲水涂层组成。在家蚕中,纤维核心包含三种蛋白质:重链丝心蛋白(Fib-H)、轻链丝心蛋白 (Fib-L) 和纤维六聚体蛋白 (Fhx,以前称为 P25)。为了进一步了解 Fhx 的作用,我们使用转录激活因子样效应核酸酶 (TALEN) 诱变并制备了Fhx 中具有无效突变的纯合系基因。我们对茧形态和蚕丝质量的表征表明突变几乎没有影响。然而,对晚龄中期突变幼虫后部丝腺 (PSG) 中分泌细胞的详细检查揭示了内质网形态的暂时变化。我们还观察到储存在Fhx的 PSG 腔中的丝心蛋白分泌小球的形态差异突变体,这表明它们的丝心蛋白复合物的溶解度略低。最后,我们进行了基于 LC-MS 的定量蛋白质组学分析,比较了突变体和野生型 (wt) 茧蛋白,发现高丰度的 16 kDa 分泌蛋白可能与丝心蛋白的溶解度有关。总体而言,我们的研究表明,虽然 Fhx 对于丝的形成是可有可无的,但它有助于细胞内运输过程中丝心蛋白复合物的稳定性,并影响 PSG 腔中丝心蛋白分泌小球的形态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号