当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative rearrangement of 3-aryl-1H-benzo[f]chromenes into 2-aroyl-1,2-dihydronaphtho[2,1-b]furans
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-06-05 , DOI: 10.1007/s10593-021-02949-9 Kirill S. Korzhenko , Dmitry V. Osipov , Vitaly А. Osyanin , Maxim R. Demidov , Yuri N. Klimochkin
中文翻译:

3-芳基-1H-苯并[f]色烯氧化重排为2-芳酰基-1,2-二氢萘[2,1-b]呋喃
更新日期:2021-06-05
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-06-05 , DOI: 10.1007/s10593-021-02949-9 Kirill S. Korzhenko , Dmitry V. Osipov , Vitaly А. Osyanin , Maxim R. Demidov , Yuri N. Klimochkin
|
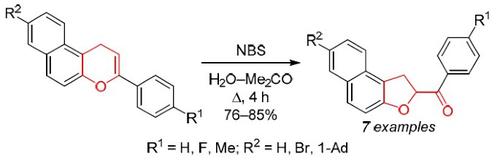
By the reaction of 3-aryl-1H-benzo[f]chromenes with N-bromosuccinimide in aqueous acetone, a series of 2-aroyl-1,2-dihydronaphtho-[2,1-b]furans unsubstituted at the C-1 position were obtained as a result of formal addition of hypobromous acid to the pyran fragment and intramolecular nucleophilic substitution. This reaction is a rare example of the ring constriction in 1H-benzo[f]chromene series.
中文翻译:

3-芳基-1H-苯并[f]色烯氧化重排为2-芳酰基-1,2-二氢萘[2,1-b]呋喃
通过 3-芳基-1 H-苯并[ f ]色烯与N-溴代琥珀酰亚胺在丙酮水溶液中的反应,一系列 2-芳酰基-1,2-二氢萘基-[2,1- b ]呋喃在 C-作为次溴酸正式加成到吡喃片段和分子内亲核取代的结果,获得了第 1 个位置。该反应是 1 H-苯并[ f ] 色烯系列中缩环的罕见例子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号