当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convenient synthesis of imidazo[1,5-a]pyrimidine derivatives and their unusual recyclization into 3H-imidazo[4,5-b]pyridine derivatives
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-06-05 , DOI: 10.1007/s10593-021-02942-2 Valery S. Tolkunov , Andrew S. Tolkunov , Olga V. Smirnova , Sergei V. Tolkunov
中文翻译:

咪唑并[1,5-a]嘧啶衍生物的简便合成及其不寻常的再环化成3H-咪唑并[4,5-b]吡啶衍生物
更新日期:2021-06-05
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-06-05 , DOI: 10.1007/s10593-021-02942-2 Valery S. Tolkunov , Andrew S. Tolkunov , Olga V. Smirnova , Sergei V. Tolkunov
|
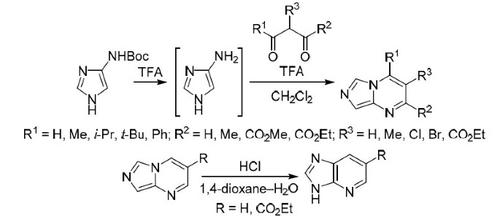
New derivatives of imidazo[1,5-a]pyrimidine have been synthesized by cyclization of in situ generated 1H-imidazol-4(5)-amine with 1,3-diketones or malondialdehyde derivatives. Utilization of asymmetrical 1,3-diketones leads to the formation of a mixture of regioisomers. The discovered conversion of imidazo[1,5-a]pyrimidine core into 3H-imidazo[4,5-b]pyridine that takes place only under acidic conditions can be considered as a new version of Dimroth rearrangement involving cleavage of C–N bond and formation of C–C bond.
中文翻译:

咪唑并[1,5-a]嘧啶衍生物的简便合成及其不寻常的再环化成3H-咪唑并[4,5-b]吡啶衍生物
咪唑并[1,5- a ]嘧啶的新衍生物是通过原位生成的1H-咪唑-4(5)-胺与1,3-二酮或丙二醛衍生物环化而合成的。不对称 1,3-二酮的使用导致区域异构体混合物的形成。咪唑并发现转化[1,5-一个]嘧啶核成3 ħ -咪唑并[4,5- b ]吡啶,只有在酸性条件下可以被认为是迪姆罗的新版本发生重排涉及C-N裂解键和 C-C 键的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号