Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The ESCRT-III complex contributes to macromitophagy in yeast
Traffic ( IF 3.6 ) Pub Date : 2021-06-05 , DOI: 10.1111/tra.12805 Zulin Wu 1 , Haiqian Xu 1 , Junze Liu 1 , Fan Zhou 1 , Yongheng Liang 1
Traffic ( IF 3.6 ) Pub Date : 2021-06-05 , DOI: 10.1111/tra.12805 Zulin Wu 1 , Haiqian Xu 1 , Junze Liu 1 , Fan Zhou 1 , Yongheng Liang 1
Affiliation
|
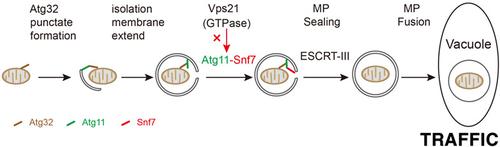
Mitochondria play important roles in energy generation and homeostasis maintenance in eukaryotic cells. The damaged or superfluous mitochondria can be nonselectively or selectively removed through the autophagy/lysosome pathway, which was referred as mitophagy. According to the molecular machinery for degrading mitochondria, the selectively removed mitochondria can occur through macromitophagy or micromitophagy. In this study, we show that the endosomal sorting complex required for transport III (ESCRT-III) in budding yeast regulates macromitophagy induced by nitrogen starvation, but not by the post-logarithmic phase growth in lactate medium by monitoring a mitochondrial marker, Om45. Firstly, loss of ESCRT-III subunit Snf7 or Vps4-Vta1 complex subunit Vps4, two representative subunits of the ESCRT complex, suppresses the delivery and degradation of Om45-GFP to vacuoles. Secondly, we show that the mitochondrial marker Om45 and mitophagy receptor Atg32 accumulate on autophagosomes marked with Atg8 (mitophagosomes, MPs) in ESCRT mutants. Moreover, the protease-protection assay indicates that Snf7 and Vps4 are involved in MP closure. Finally, Snf7 interacts with Atg11, which was detected by two ways, glutathione-S-transferase (GST) pulldown and bimolecular fluorescence complementation (BiFC) assay, and this BiFC interaction happens on mitochondrial reticulum. Therefore, we proposed that the ESCRT-III machinery mediates nitrogen starvation-induced macromitophagy by the interaction between Snf7 and Atg11 so that Snf7 is recruited to Atg32-marked MPs by the known Atg11–Atg32 interaction to seal them. These results reveal that the ESCRT-III complex plays a new role in yeast on macromitophagy.
中文翻译:

ESCRT-III 复合物有助于酵母中的巨噬细胞
线粒体在真核细胞的能量产生和体内平衡维持中起着重要作用。受损或多余的线粒体可以通过自噬/溶酶体途径非选择性或选择性去除,这被称为线粒体自噬。根据降解线粒体的分子机制,选择性去除的线粒体可以通过巨线粒体自噬或微线粒体自噬发生。在这项研究中,我们显示出芽酵母中转运 III (ESCRT-III) 所需的内体分选复合物可调节由氮饥饿引起的巨噬细胞,但不能通过监测线粒体标记物 Om45 来调节乳酸培养基中的后对数期生长。首先,ESCRT-III 亚基 Snf7 或 Vps4-Vta1 复合亚基 Vps4 丢失,这是 ESCRT 复合体的两个代表性亚基,抑制 Om45-GFP 向液泡的传递和降解。其次,我们表明线粒体标记物 Om45 和线粒体自噬受体 Atg32 在 ESCRT 突变体中以 Atg8(mitophagosomes,MPs)标记的自噬体上积累。此外,蛋白酶保护测定表明 Snf7 和 Vps4 参与 MP 关闭。最后,Snf7 与 Atg11 相互作用,可通过两种方式检测,即谷胱甘肽-S-转移酶 (GST) 下拉和双分子荧光互补 (BiFC) 测定,这种 BiFC 相互作用发生在线粒体网中。因此,我们提出 ESCRT-III 机制通过 Snf7 和 Atg11 之间的相互作用介导氮饥饿诱导的巨噬细胞,以便通过已知的 Atg11-Atg32 相互作用将 Snf7 募集到 Atg32 标记的 MP 以密封它们。
更新日期:2021-07-27
中文翻译:

ESCRT-III 复合物有助于酵母中的巨噬细胞
线粒体在真核细胞的能量产生和体内平衡维持中起着重要作用。受损或多余的线粒体可以通过自噬/溶酶体途径非选择性或选择性去除,这被称为线粒体自噬。根据降解线粒体的分子机制,选择性去除的线粒体可以通过巨线粒体自噬或微线粒体自噬发生。在这项研究中,我们显示出芽酵母中转运 III (ESCRT-III) 所需的内体分选复合物可调节由氮饥饿引起的巨噬细胞,但不能通过监测线粒体标记物 Om45 来调节乳酸培养基中的后对数期生长。首先,ESCRT-III 亚基 Snf7 或 Vps4-Vta1 复合亚基 Vps4 丢失,这是 ESCRT 复合体的两个代表性亚基,抑制 Om45-GFP 向液泡的传递和降解。其次,我们表明线粒体标记物 Om45 和线粒体自噬受体 Atg32 在 ESCRT 突变体中以 Atg8(mitophagosomes,MPs)标记的自噬体上积累。此外,蛋白酶保护测定表明 Snf7 和 Vps4 参与 MP 关闭。最后,Snf7 与 Atg11 相互作用,可通过两种方式检测,即谷胱甘肽-S-转移酶 (GST) 下拉和双分子荧光互补 (BiFC) 测定,这种 BiFC 相互作用发生在线粒体网中。因此,我们提出 ESCRT-III 机制通过 Snf7 和 Atg11 之间的相互作用介导氮饥饿诱导的巨噬细胞,以便通过已知的 Atg11-Atg32 相互作用将 Snf7 募集到 Atg32 标记的 MP 以密封它们。











































 京公网安备 11010802027423号
京公网安备 11010802027423号