当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of brain-penetrant picolinamide derived leucine-rich repeat kinase 2 (LRRK2) inhibitors
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2021-6-4 , DOI: 10.1039/d1md00097g Anmol Gulati 1 , Charles S Yeung 1 , Blair Lapointe 1 , Solomon D Kattar 1 , Hakan Gunaydin 1 , Jack D Scott 2 , Kaleen K Childers 1 , Joey L Methot 1 , Vladimir Simov 1 , Ravi Kurukulasuriya 1 , Barbara Pio 2 , Greg J Morriello 2 , Ping Liu 2 , Haiqun Tang 2 , Santhosh Neelamkavil 2 , Harold B Wood 2 , Vanessa L Rada 3 , Michael J Ardolino 1 , Xin Cindy Yan 1 , Rachel Palte 1 , Karin Otte 1 , Robert Faltus 1 , Janice Woodhouse 1 , Laxminarayan G Hegde 1 , Paul Ciaccio 1 , Ellen C Minnihan 1 , Erin F DiMauro 1 , Matthew J Fell 1 , Peter H Fuller 1 , J Michael Ellis 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2021-6-4 , DOI: 10.1039/d1md00097g Anmol Gulati 1 , Charles S Yeung 1 , Blair Lapointe 1 , Solomon D Kattar 1 , Hakan Gunaydin 1 , Jack D Scott 2 , Kaleen K Childers 1 , Joey L Methot 1 , Vladimir Simov 1 , Ravi Kurukulasuriya 1 , Barbara Pio 2 , Greg J Morriello 2 , Ping Liu 2 , Haiqun Tang 2 , Santhosh Neelamkavil 2 , Harold B Wood 2 , Vanessa L Rada 3 , Michael J Ardolino 1 , Xin Cindy Yan 1 , Rachel Palte 1 , Karin Otte 1 , Robert Faltus 1 , Janice Woodhouse 1 , Laxminarayan G Hegde 1 , Paul Ciaccio 1 , Ellen C Minnihan 1 , Erin F DiMauro 1 , Matthew J Fell 1 , Peter H Fuller 1 , J Michael Ellis 1
Affiliation
|
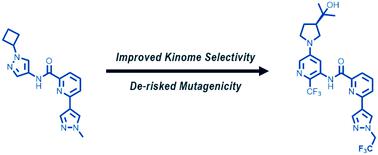
The discovery of potent, kinome selective, brain penetrant LRRK2 inhibitors is the focus of extensive research seeking new, disease-modifying treatments for Parkinson's disease (PD). Herein, we describe the discovery and evolution of a picolinamide-derived lead series. Our initial optimization efforts aimed at improving the potency and CLK2 off-target selectivity of compound 1 by modifying the heteroaryl C–H hinge and linker regions. This resulted in compound 12 which advanced deep into our research operating plan (ROP) before heteroaryl aniline metabolite 14 was characterized as Ames mutagenic, halting its progression. Strategic modifications to our ROP were made to enable early de-risking of putative aniline metabolites or hydrolysis products for mutagenicity in Ames. This led to the discovery of 3,5-diaminopyridine 15 and 4,6-diaminopyrimidine 16 as low risk for mutagenicity (defined by a 3-strain Ames negative result). Analysis of key matched molecular pairs 17 and 18 led to the prioritization of the 3,5-diaminopyridine sub-series for further optimization due to enhanced rodent brain penetration. These efforts culminated in the discovery of ethyl trifluoromethyl pyrazole 23 with excellent LRRK2 potency and expanded selectivity versus off-target CLK2.
中文翻译:

脑渗透性吡啶酰胺衍生的富含亮氨酸重复激酶 2 (LRRK2) 抑制剂的优化
强效、激酶组选择性、脑渗透性 LRRK2 抑制剂的发现是寻求新的、改善帕金森病 (PD) 疾病的治疗方法的广泛研究的重点。在这里,我们描述了吡啶酰胺衍生的铅系列的发现和演变。我们最初的优化工作旨在通过修饰杂芳基 C-H 铰链和接头区域来提高化合物1的效力和 CLK2 脱靶选择性。这导致化合物12在杂芳基苯胺代谢物14之前深入我们的研究操作计划 (ROP)被定性为 Ames 致突变性,阻止其进展。对我们的 ROP 进行了战略性修改,以便尽早降低 Ames 中假定的苯胺代谢物或水解产物的致突变性风险。这导致发现 3,5-二氨基吡啶15和 4,6-二氨基嘧啶16具有低致突变风险(定义为 3 株 Ames 阴性结果)。对关键匹配分子对17和18的分析导致优先考虑 3,5-二氨基吡啶子系列,以进一步优化啮齿动物脑渗透。这些努力最终发现乙基三氟甲基吡唑23具有出色的 LRRK2 效力和扩大的选择性与偏离目标的 CLK2。
更新日期:2021-06-04
中文翻译:

脑渗透性吡啶酰胺衍生的富含亮氨酸重复激酶 2 (LRRK2) 抑制剂的优化
强效、激酶组选择性、脑渗透性 LRRK2 抑制剂的发现是寻求新的、改善帕金森病 (PD) 疾病的治疗方法的广泛研究的重点。在这里,我们描述了吡啶酰胺衍生的铅系列的发现和演变。我们最初的优化工作旨在通过修饰杂芳基 C-H 铰链和接头区域来提高化合物1的效力和 CLK2 脱靶选择性。这导致化合物12在杂芳基苯胺代谢物14之前深入我们的研究操作计划 (ROP)被定性为 Ames 致突变性,阻止其进展。对我们的 ROP 进行了战略性修改,以便尽早降低 Ames 中假定的苯胺代谢物或水解产物的致突变性风险。这导致发现 3,5-二氨基吡啶15和 4,6-二氨基嘧啶16具有低致突变风险(定义为 3 株 Ames 阴性结果)。对关键匹配分子对17和18的分析导致优先考虑 3,5-二氨基吡啶子系列,以进一步优化啮齿动物脑渗透。这些努力最终发现乙基三氟甲基吡唑23具有出色的 LRRK2 效力和扩大的选择性与偏离目标的 CLK2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号