Structure ( IF 5.7 ) Pub Date : 2021-06-02 , DOI: 10.1016/j.str.2021.05.011 Andreas Haahr Larsen 1 , Mark S P Sansom 1
|
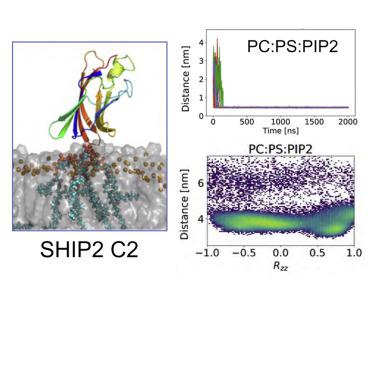
C2 domains facilitate protein interactions with lipid bilayers in either a Ca2+-dependent or -independent manner. We used molecular dynamics (MD) simulations to explore six Ca2+-independent C2 domains, from KIBRA, PI3KC2α, RIM2, PTEN, SHIP2, and Smurf2. In coarse-grained MD simulations these C2 domains formed transient interactions with zwitterionic bilayers, compared with longer-lived interactions with anionic bilayers containing phosphatidylinositol bisphosphate (PIP2). Type I C2 domains bound non-canonically via the front, back, or side of the β sandwich, whereas type II C2 domains bound canonically, via the top loops. C2 domains interacted strongly with membranes containing PIP2, causing bound anionic lipids to cluster around the protein. Binding modes were refined via atomistic simulations. For PTEN and SHIP2, CG simulations of their phosphatase plus C2 domains with PIP2-containing bilayers were also performed, and the roles of the two domains in membrane localization compared. These studies establish a simulation protocol for membrane-recognition proteins.
中文翻译:

Ca2+非依赖性 C2 结构域与脂质膜的结合:多尺度分子动力学研究
C2结构域以Ca 2+依赖性或非依赖性方式促进蛋白质与脂质双层的相互作用。我们使用分子动力学 (MD) 模拟来探索来自 KIBRA、PI3KC2α、RIM2、PTEN、SHIP2 和 Smurf2 的六个 Ca 2+独立 C2 结构域。在粗粒度 MD 模拟中,这些 C2 结构域与两性离子双层形成瞬时相互作用,而与含有磷脂酰肌醇二磷酸 (PIP 2 ) 的阴离子双层的相互作用寿命更长。I 型 C2 结构域通过 β 夹层的正面、背面或侧面非规范结合,而 II 型 C2 结构域通过顶部环规范结合。C2 结构域与含有 PIP 2的膜强烈相互作用,导致结合的阴离子脂质聚集在蛋白质周围。通过原子模拟改进了结合模式。对于 PTEN 和 SHIP2,还对它们的磷酸酶加 C2 结构域和含有 PIP 2的双层进行了 CG 模拟,并比较了这两个结构域在膜定位中的作用。这些研究建立了膜识别蛋白的模拟协议。



























 京公网安备 11010802027423号
京公网安备 11010802027423号