当前位置:
X-MOL 学术
›
Arch. Insect Biochem. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The acetylation modification regulates the stability of Bm30K-15 protein and its mechanism in silkworm, Bombyx mori
Archives of Insect Biochemistry and Physiology ( IF 1.5 ) Pub Date : 2021-06-01 , DOI: 10.1002/arch.21823 Jiao Lv 1 , Shouliang Li 1 , Yue Liu 2 , Zihan Sun 1 , Dan Wang 1 , Zhengying You 1 , Caiying Jiang 1 , Qing Sheng 1 , Zuoming Nie 1
Archives of Insect Biochemistry and Physiology ( IF 1.5 ) Pub Date : 2021-06-01 , DOI: 10.1002/arch.21823 Jiao Lv 1 , Shouliang Li 1 , Yue Liu 2 , Zihan Sun 1 , Dan Wang 1 , Zhengying You 1 , Caiying Jiang 1 , Qing Sheng 1 , Zuoming Nie 1
Affiliation
|
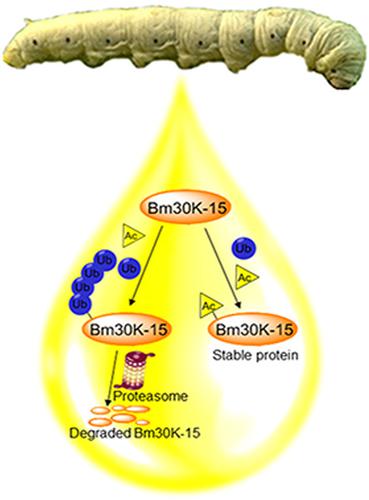
The 30 K proteins are the major silkworm hemolymph proteins and are involved in a variety of physiological processes, such as nutrient and energy storage, embryogenesis, immune response, and inhibition of apoptosis. The Bm30K-15 protein is one of the 30 K proteins and is abundant in the hemolymph of fifth instar silkworm larva. We previously found that the Bm30K-15 protein can be acetylated. In the present study, we found that acetylation can improve the protein stability of Bm30K-15. Further exploration confirmed that the increase in protein stability by acetylation was caused by competition between acetylation and ubiquitination. In summary, these findings aim to provide insight into the effect of acetylation modification on the protein level and stability of the Bm30K-15 and the possible molecular mechanism of its existence in silkworm, Bombyx mori.
中文翻译:

乙酰化修饰调控家蚕 Bm30K-15 蛋白的稳定性及其机制
30 K 蛋白是家蚕主要的血淋巴蛋白,参与多种生理过程,如营养和能量储存、胚胎发生、免疫反应和细胞凋亡抑制。Bm30K-15 蛋白是 30 K 蛋白之一,在五龄蚕幼虫的血淋巴中含量丰富。我们以前发现 Bm30K-15 蛋白可以被乙酰化。在本研究中,我们发现乙酰化可以提高 Bm30K-15 的蛋白质稳定性。进一步的探索证实,乙酰化对蛋白质稳定性的增加是乙酰化与泛素化竞争引起的。总之,这些发现旨在深入了解乙酰化修饰对 Bm30K-15 蛋白质水平和稳定性的影响及其在家蚕中存在的可能分子机制,家蚕。
更新日期:2021-06-23
中文翻译:

乙酰化修饰调控家蚕 Bm30K-15 蛋白的稳定性及其机制
30 K 蛋白是家蚕主要的血淋巴蛋白,参与多种生理过程,如营养和能量储存、胚胎发生、免疫反应和细胞凋亡抑制。Bm30K-15 蛋白是 30 K 蛋白之一,在五龄蚕幼虫的血淋巴中含量丰富。我们以前发现 Bm30K-15 蛋白可以被乙酰化。在本研究中,我们发现乙酰化可以提高 Bm30K-15 的蛋白质稳定性。进一步的探索证实,乙酰化对蛋白质稳定性的增加是乙酰化与泛素化竞争引起的。总之,这些发现旨在深入了解乙酰化修饰对 Bm30K-15 蛋白质水平和稳定性的影响及其在家蚕中存在的可能分子机制,家蚕。











































 京公网安备 11010802027423号
京公网安备 11010802027423号