当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acrylonitrile Conversion on Metal Cathodes: How Surface Adsorption Determines the Reduction Pathways
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-06-02 , DOI: 10.1021/acs.iecr.1c00813 Shutao Wu 1 , Hongliang Zhang 1 , Xun Huang 1 , Qiang Liao 2 , Zidong Wei 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-06-02 , DOI: 10.1021/acs.iecr.1c00813 Shutao Wu 1 , Hongliang Zhang 1 , Xun Huang 1 , Qiang Liao 2 , Zidong Wei 1
Affiliation
|
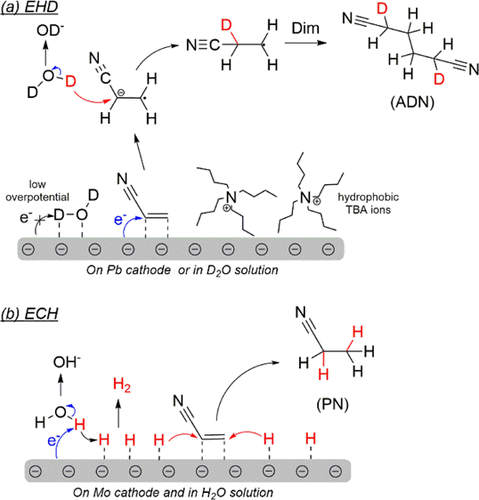
Electrochemical dimerization of acrylonitrile (AN) on metal cathodes is a simple and green method for manufacturing adiponitrile (ADN), the raw material for nylon 66. However, the current controversial mechanisms hinder the development of more active catalysts. Here, from a combination of experiments and theoretical calculations, we found that the production rate of ADN and byproducts is highly related to the surface adsorption state. High ADN selectivity is obtained only when the surface is mainly covered by AN. Substitution of AN with hydrogen will promote surface hydrogenation to form propionitrile (PN), and a full coverage of hydrogen exclusively leads to hydrogen evolution. An H/D isotopic experiment revealed that the hydrogen adsorbed on an electrode mainly participates in the saturation of AN to PN, while the proton in an aqueous phase contributes to the dimerization of AN to ADN. These results can provide certain guidance for catalyst design of an AN electroreduction reaction.
中文翻译:

金属阴极上的丙烯腈转化:表面吸附如何决定还原途径
丙烯腈(AN)在金属阴极上的电化学二聚是制备己二腈(ADN)(尼龙66的原料)的一种简单且绿色的方法。然而,目前有争议的机制阻碍了更具活性的催化剂的开发。在这里,结合实验和理论计算,我们发现 ADN 和副产物的产率与表面吸附状态高度相关。只有当表面主要被 AN 覆盖时才能获得高 ADN 选择性。用氢取代 AN 将促进表面氢化形成丙腈 (PN),而氢的完全覆盖只会导致析氢。H/D 同位素实验表明,吸附在电极上的氢主要参与 AN 到 PN 的饱和,而水相中的质子有助于 AN 二聚化为 ADN。这些结果可为AN电还原反应的催化剂设计提供一定的指导。
更新日期:2021-06-17
中文翻译:

金属阴极上的丙烯腈转化:表面吸附如何决定还原途径
丙烯腈(AN)在金属阴极上的电化学二聚是制备己二腈(ADN)(尼龙66的原料)的一种简单且绿色的方法。然而,目前有争议的机制阻碍了更具活性的催化剂的开发。在这里,结合实验和理论计算,我们发现 ADN 和副产物的产率与表面吸附状态高度相关。只有当表面主要被 AN 覆盖时才能获得高 ADN 选择性。用氢取代 AN 将促进表面氢化形成丙腈 (PN),而氢的完全覆盖只会导致析氢。H/D 同位素实验表明,吸附在电极上的氢主要参与 AN 到 PN 的饱和,而水相中的质子有助于 AN 二聚化为 ADN。这些结果可为AN电还原反应的催化剂设计提供一定的指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号