当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aqueous Photochemistry of 2-Methyltetrol and Erythritol as Sources of Formic Acid and Acetic Acid in the Atmosphere
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-06-02 , DOI: 10.1021/acsearthspacechem.1c00107 James D. Cope 1 , Karizza A. Abellar 2 , Kelvin H. Bates 1, 3 , Xuan Fu 1 , Tran B. Nguyen 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-06-02 , DOI: 10.1021/acsearthspacechem.1c00107 James D. Cope 1 , Karizza A. Abellar 2 , Kelvin H. Bates 1, 3 , Xuan Fu 1 , Tran B. Nguyen 1
Affiliation
|
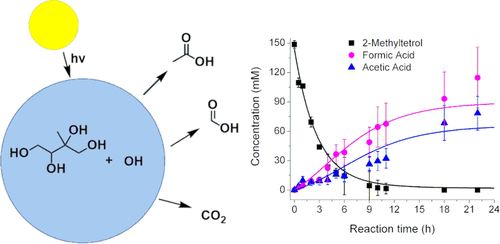
Atmospheric formic acid (FA) and acetic acid (AA) mixing ratios are often underestimated in atmospheric models, particularly over areas with high biogenic influence. We investigated the aqueous hydroxyl radical (OH) oxidation of 2-methyltetrol, one of the largest components of secondary organic aerosols (SOAs) that are produced from the oxidation of isoprene, and compare its chemistry to the non-methylated C4 polyol analogue, erythritol. We studied the kinetics and reaction products of the aqueous 2-methyltetrol (2-MT) + OH and erythritol (E) + OH reactions using 1H and 13C nuclear magnetic resonance spectroscopy and high-performance liquid chromatography coupled with high-resolution mass spectrometry. We found that the aqueous oxidation of aliphatic alcohols, such as E and 2-MT, are strong sources of small acids. Nearly all 2-MT is converted to FA, AA, and carbon dioxide (CO2) under atmospherically relevant OH exposures. Suppression of volatile acid partitioning into the gas phase increased the observed yields of volatile products in solution by up to 80%, as quantified by experiments with low headspace. The influence of solution pH on the yields of FA and AA (or their carboxylates) was also investigated in the range of pH 2–9 for the 2-MT + OH reaction. Solution pH strongly influenced the concentrations of FA and AA via their gas–aqueous partitioning, gross production yields, and radical-induced decarboxylation reactions. The data are adequately reproduced with a kinetic model; however, different reaction mechanisms are needed for the low and high pH chemistries. Fewer stable reaction intermediates were observed for 2-MT compared to E and at high pH compared to low pH, providing insight into the decomposition pathways of 2-MT. On the basis of the substantial production yields and partitioning of FA and AA in the aqueous photooxidation of 2-methyltetrol, aqueous aging of isoprene-derived SOA may contribute to FA and AA emissions to the atmosphere that are currently missing from models.
中文翻译:

作为大气中甲酸和乙酸来源的 2-甲基四醇和赤藓糖醇的水性光化学
在大气模型中,大气甲酸 (FA) 和乙酸 (AA) 的混合比通常被低估,特别是在具有高生物成因影响的地区。我们研究了 2-甲基四醇(异戊二烯氧化产生的二次有机气溶胶 (SOA) 的最大成分之一)的水性羟基自由基 (OH) 氧化,并将其化学性质与非甲基化的 C 4多元醇类似物进行了比较,赤藓糖醇。我们使用1 H 和13研究了水性 2-甲基四醇 (2-MT) + OH 和赤藓糖醇 (E) + OH 反应的动力学和反应产物C 核磁共振波谱和高效液相色谱与高分辨率质谱联用。我们发现脂肪醇(如 E 和 2-MT)的水氧化是小酸的强来源。几乎所有 2-MT 都转化为 FA、AA 和二氧化碳(CO 2) 在与大气相关的 OH 暴露下。抑制分配到气相中的挥发性酸使观察到的溶液中挥发性产物的产量增加了 80%,这通过低顶空实验进行了量化。对于 2-MT + OH 反应,在 pH 2-9 范围内还研究了溶液 pH 对 FA 和 AA(或它们的羧酸盐)产率的影响。溶液 pH 值通过它们的气-水分配、总产率和自由基诱导的脱羧反应强烈影响 FA 和 AA 的浓度。用动力学模型充分再现了数据;然而,低和高 pH 值化学需要不同的反应机制。与 E 相比,在高 pH 下观察到 2-MT 的稳定反应中间体较少,与低 pH 相比,深入了解 2-MT 的分解途径。基于 FA 和 AA 在 2-甲基四醇的水性光氧化中的大量生产和分配,异戊二烯衍生的 SOA 的水性老化可能会导致 FA 和 AA 排放到大气中,而这些排放是目前模型中缺失的。
更新日期:2021-06-17
中文翻译:

作为大气中甲酸和乙酸来源的 2-甲基四醇和赤藓糖醇的水性光化学
在大气模型中,大气甲酸 (FA) 和乙酸 (AA) 的混合比通常被低估,特别是在具有高生物成因影响的地区。我们研究了 2-甲基四醇(异戊二烯氧化产生的二次有机气溶胶 (SOA) 的最大成分之一)的水性羟基自由基 (OH) 氧化,并将其化学性质与非甲基化的 C 4多元醇类似物进行了比较,赤藓糖醇。我们使用1 H 和13研究了水性 2-甲基四醇 (2-MT) + OH 和赤藓糖醇 (E) + OH 反应的动力学和反应产物C 核磁共振波谱和高效液相色谱与高分辨率质谱联用。我们发现脂肪醇(如 E 和 2-MT)的水氧化是小酸的强来源。几乎所有 2-MT 都转化为 FA、AA 和二氧化碳(CO 2) 在与大气相关的 OH 暴露下。抑制分配到气相中的挥发性酸使观察到的溶液中挥发性产物的产量增加了 80%,这通过低顶空实验进行了量化。对于 2-MT + OH 反应,在 pH 2-9 范围内还研究了溶液 pH 对 FA 和 AA(或它们的羧酸盐)产率的影响。溶液 pH 值通过它们的气-水分配、总产率和自由基诱导的脱羧反应强烈影响 FA 和 AA 的浓度。用动力学模型充分再现了数据;然而,低和高 pH 值化学需要不同的反应机制。与 E 相比,在高 pH 下观察到 2-MT 的稳定反应中间体较少,与低 pH 相比,深入了解 2-MT 的分解途径。基于 FA 和 AA 在 2-甲基四醇的水性光氧化中的大量生产和分配,异戊二烯衍生的 SOA 的水性老化可能会导致 FA 和 AA 排放到大气中,而这些排放是目前模型中缺失的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号