当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Carbon Dioxide in Carboxylation Reaction Mixtures
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-06-01 , DOI: 10.1021/acs.iecr.1c00430 Pallavi Bobba 1, 2 , Hongda Zhu 1 , William Kirk Snavely 1 , Dupeng Liu 1 , Bala Subramaniam 1, 2 , Raghunath Vitthal Chaudhari 1, 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-06-01 , DOI: 10.1021/acs.iecr.1c00430 Pallavi Bobba 1, 2 , Hongda Zhu 1 , William Kirk Snavely 1 , Dupeng Liu 1 , Bala Subramaniam 1, 2 , Raghunath Vitthal Chaudhari 1, 2
Affiliation
|
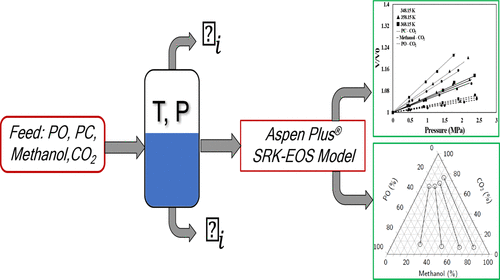
The solubility of carbon dioxide (CO2) in pure solvents such as methanol, propylene carbonate (PC), propylene oxide (PO), and their mixtures was experimentally measured at different temperatures (348.2–368.2 K) and pressures (0.5–2.0 MPa). These data are required for analysis of rate processes in carboxylation of PO to PC, an industrially important reaction for the manufacture of alkyl carbonates. At higher pressures, increasing volumetric expansion of the solvents by CO2 was noted for both binary and ternary mixtures of the solvents with CO2. The Soave–Redlich–Kwong (SRK) equation of state predictions of the expansions were generally satisfactory for systems involving PC, methanol, and CO2 (±2–7% AAPD from experimental values). However, the deviation (±5–17% AAPD) was generally greater in systems involving PO as a solvent. The experimental volumetric expansion data were used to accurately represent the liquid volumes in the estimation of CO2 mole fractions in neat solvents. Similar to volume expansion trends, the SRK predictions of CO2 mole fractions in PC and methanol (±3–9% AAPD from experimental values) were also satisfactory, while the deviation was greater for the predicted CO2 mole fraction in PO (±28–33% AAPD). For the ternary (PO–PC–CO2, PO–methanol–CO2, PC–methanol–CO2) and quaternary (PO–PC–methanol–CO2) mixtures, vapor–liquid equilibrium (VLE) data were generated using the AspenPlus SRK-EOS model via flash calculations based on composition of the liquid mixture charged and the experimentally measured CO2 uptake at equilibrium.
中文翻译:

二氧化碳在羧化反应混合物中的溶解度
二氧化碳 (CO 2 ) 在甲醇、碳酸丙烯酯 (PC)、环氧丙烷 (PO) 及其混合物等纯溶剂中的溶解度是在不同温度 (348.2–368.2 K) 和压力 (0.5–2.0 MPa) 下通过实验测量的)。这些数据对于分析 PO 羧化为 PC 的速率过程是必需的,PC 是生产碳酸烷基酯的工业上重要的反应。在更高的压力下,对于溶剂与CO 2 的二元和三元混合物,注意到CO 2引起的溶剂体积膨胀增加。Soave-Redlich-Kwong (SRK) 方程的膨胀状态预测对于涉及 PC、甲醇和 CO 2 的系统通常是令人满意的(来自实验值的 ±2–7% AAPD)。然而,在涉及 PO 作为溶剂的系统中,偏差 (±5–17% AAPD) 通常更大。在估算纯溶剂中的 CO 2摩尔分数时,实验体积膨胀数据用于准确表示液体体积。与体积膨胀趋势相似,PC 和甲醇中 CO 2摩尔分数的 SRK 预测(实验值的±3-9% AAPD)也令人满意,而预测的PO 中CO 2摩尔分数的偏差更大(±28 –33% AAPD)。对于三元(PO-PC-CO 2、PO-甲醇-CO 2、PC-甲醇-CO 2)和四元(PO-PC-甲醇-CO 2) 混合物,气液平衡 (VLE) 数据是使用 AspenPlus SRK-EOS 模型通过闪蒸计算生成的,该计算基于所装液体混合物的组成和实验测量的平衡时的CO 2吸收。
更新日期:2021-06-17
中文翻译:

二氧化碳在羧化反应混合物中的溶解度
二氧化碳 (CO 2 ) 在甲醇、碳酸丙烯酯 (PC)、环氧丙烷 (PO) 及其混合物等纯溶剂中的溶解度是在不同温度 (348.2–368.2 K) 和压力 (0.5–2.0 MPa) 下通过实验测量的)。这些数据对于分析 PO 羧化为 PC 的速率过程是必需的,PC 是生产碳酸烷基酯的工业上重要的反应。在更高的压力下,对于溶剂与CO 2 的二元和三元混合物,注意到CO 2引起的溶剂体积膨胀增加。Soave-Redlich-Kwong (SRK) 方程的膨胀状态预测对于涉及 PC、甲醇和 CO 2 的系统通常是令人满意的(来自实验值的 ±2–7% AAPD)。然而,在涉及 PO 作为溶剂的系统中,偏差 (±5–17% AAPD) 通常更大。在估算纯溶剂中的 CO 2摩尔分数时,实验体积膨胀数据用于准确表示液体体积。与体积膨胀趋势相似,PC 和甲醇中 CO 2摩尔分数的 SRK 预测(实验值的±3-9% AAPD)也令人满意,而预测的PO 中CO 2摩尔分数的偏差更大(±28 –33% AAPD)。对于三元(PO-PC-CO 2、PO-甲醇-CO 2、PC-甲醇-CO 2)和四元(PO-PC-甲醇-CO 2) 混合物,气液平衡 (VLE) 数据是使用 AspenPlus SRK-EOS 模型通过闪蒸计算生成的,该计算基于所装液体混合物的组成和实验测量的平衡时的CO 2吸收。











































 京公网安备 11010802027423号
京公网安备 11010802027423号