Progress in Neuro-Psychopharmacology and Biological Psychiatry ( IF 5.6 ) Pub Date : 2021-06-01 , DOI: 10.1016/j.pnpbp.2021.110368 Muhammed A Saad 1 , Maha A E Ahmed 2 , Norhan N Elbadawy 2 , Noha F Abdelkader 3
|
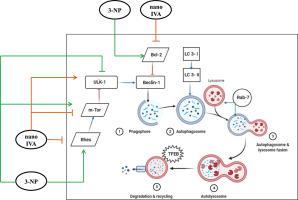
Huntington's disease (HD) is characterized by abnormal involuntary movements together with cognitive impairment and disrupted mood changes. 3-nitropropionic acid (3-NP) is one of the chemo-toxic models used to address the striatal neurotoxicity pattern encountered in HD. This study aims to explain the neuroprotective effect of nano-formulated ivabradine (nano IVA) in enhancing behavioral changes related to 3-NP model and to identify the involvement of ras homolog enriched striatum (Rhes)/mammalian target of rapamycin (m-Tor) mediated autophagy pathway. Rats were divided into 6 groups, the first 3 groups received saline (control), ivabradine (IVA), nano IVA respectively, the fourth received a daily dose of 3-NP (20 mg/kg, s.c) for 2 weeks, the fifth received 3-NP + IVA (1 mg/kg, into the tail vein, every other day for 1 week) and the last group received 3-NP + nano IVA (1 mg/kg, i.v, every other day for 1 week). Interestingly, nano IVA reversed motor disabilities, improved memory function and overcame the psychiatric changes. It boosted expression of autophagy markers combined with down regulation of Rhes, m-Tor and b-cell lymphoma 2 protein levels. Also, it restored the normal level of neurotransmitters and myocardial function related-proteins. Histopathological examination revealed a preserved striatal structure with decreased number of darkly-degenerated neurons. In conclusion, the outcomes of this study provide a well-recognized clue for the promising neuroprotective effect of IVA and the implication of autophagy and Rhes/m-Tor pathways in the 3-NP induced HD and highlight the fact that nano formulations of IVA would be an auspicious approach in HD therapy.
中文翻译:

纳米伊伐布雷定通过调节 Rhes/m-tor 通路避免亨廷顿病大鼠模型的行为异常
亨廷顿病 (HD) 的特征是异常的不自主运动以及认知障碍和情绪变化紊乱。3-硝基丙酸 (3-NP) 是一种化学毒性模型,用于解决 HD 中遇到的纹状体神经毒性模式。本研究旨在解释纳米制剂伊伐布雷定 (nano IVA) 在增强与 3-NP 模型相关的行为变化方面的神经保护作用,并确定 ras 同源富集纹状体 (Rhes)/雷帕霉素哺乳动物靶标 (m-Tor) 的参与介导的自噬途径。大鼠分为6组,前3组分别给予生理盐水(对照)、伊伐布雷定(IVA)、纳米IVA,第四组给予每日剂量3-NP(20 mg/kg,sc)2周,第五组给予接受 3-NP + IVA(1 mg/kg,进入尾静脉,每隔一天,持续 1 周),最后一组接受 3-NP + nano IVA(1 mg/kg,iv,每隔一天,持续 1 周)。有趣的是,纳米 IVA 逆转了运动障碍,改善了记忆功能并克服了精神方面的变化。它提高了自噬标志物的表达,同时下调了 Rhes、m-Tor 和 b 细胞淋巴瘤 2 蛋白水平。此外,它恢复了神经递质和心肌功能相关蛋白的正常水平。组织病理学检查显示纹状体结构保留,深色退化的神经元数量减少。综上所述,



























 京公网安备 11010802027423号
京公网安备 11010802027423号