当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, characterization, and biological activities of some novel thienylpyrido[3′,2′:4,5]thieno[3,2-d]pyrimidines and related heterocycles
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-05-30 , DOI: 10.1002/jhet.4310 Suzan Abuelhassan 1 , Etify A.‐G. Bakhite 1 , Abdu E. Abdel‐Rahman 1 , Ahmed F. M. El‐Mahdy 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-05-30 , DOI: 10.1002/jhet.4310 Suzan Abuelhassan 1 , Etify A.‐G. Bakhite 1 , Abdu E. Abdel‐Rahman 1 , Ahmed F. M. El‐Mahdy 1
Affiliation
|
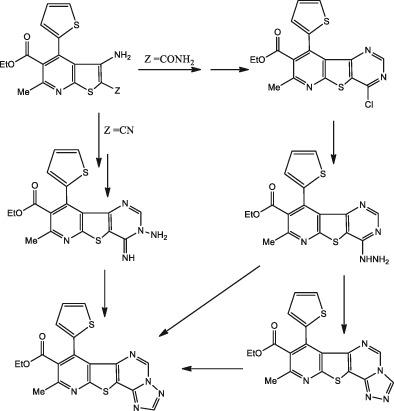
3-Cyano-5-ethoxycarbonyl-6-methyl-4-(2′-thienyl)-pyridine-2(1H)-thione (1) is synthesized and reacted with chloroacetamide or chloroacetonitrile to give 3-amino-5-ethoxycarbonyl-6-methyl-4(2′-thienyl)-thieno[2,3-b]pyridine-2-carboxamide 3a or its 2-carbonitrile analog 3b, respectively. Cyclocondensation of 3a with triethylorthoformate produced the corresponding pyridothienopyrimidineone 4, which on heating with phosphorus oxychloride gave 4-chloropyrimidine derivative 5. Compound 5 was used as key intermediate for synthesizing compounds 6, 9, 10, 11, and 12 upon treatment with some nucleophilic reagents such as thiourea, 5-phenyl-s-triazole-3(1H)-thione, piperidine, morpholine, or hydrazine hydrate, respectively. Reaction of pyridothienopyrimidinethione 6 with N-(4-tolyl)-2-chloroacetamide or ethyl bromoacetate afforded the corresponding S-substituted methylsulfanylpyrimidines 7 or 8. The condensation of 3b with triethylorthoformate gave azomethine derivative 13, which was reacted with hydrazine hydrate to give ethyl 3-amino-3,4-dihydro-4-imino-7-methyl-9-(2′-thienyl)pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidine-8-carboxylate (14). Compounds 12 and 14 were used as precursors for synthesizing other new thienylpyridothienopyrimidines as well as isomeric thienyl-s-triazolopyridothieno- pyrimidines. All synthesized compounds were characterized by elemental and spectral analyses such as IR, 1H NMR, and 13C NMR. In addition, majority of synthesized compounds were tested for their antifungal activity against five strains of fungi. Moreover, compounds 3a, 5, 6, 8, and 22 were screened for their anticancer activity against HEPG-2 and MCF-7 cell lines.
中文翻译:

一些新型噻吩并吡啶并[3',2':4,5]噻吩并[3,2-d]嘧啶和相关杂环的合成、表征和生物活性
合成 3-Cyano-5-ethoxycarbonyl-6-methyl-4-(2'-thienyl)-pyridine-2(1 H )-thion ( 1 ) 并与氯乙酰胺或氯乙腈反应生成 3-amino-5-ethoxycarbonyl -6-甲基-4(2'-噻吩基)-噻吩并[2,3 - b ]吡啶-2-甲酰胺3a或其2-腈类似物3b。的环缩合3A与原甲酸三乙酯中产生的相应pyridothienopyrimidineone 4,其上用磷酰氯中加热得到4-氯嘧啶衍生物5。化合物5作为关键中间体用于合成化合物6 , 9 ,10,11,和12一旦与亲核一些试剂如硫脲处理,5-苯基小号三唑-3(1 ħ) -硫酮,哌啶,吗啉,或水合肼,分别。吡啶并噻吩并嘧啶硫酮6与N- (4-甲苯基)-2-氯乙酰胺或溴乙酸乙酯反应得到相应的S-取代的甲基硫基嘧啶7或8。3b与原甲酸三乙酯缩合得到偶氮甲碱衍生物13, 与水合肼反应生成乙基 3-amino-3,4-dihydro-4-imino-7-methyl-9-(2'-thienyl)pyrido[3',2':4,5]thieno[ 3,2 - d ]嘧啶-8-羧酸酯( 14 )。化合物12和14用作合成其他新的噻吩基吡啶并噻吩并嘧啶以及异构噻吩基-s-三唑并吡啶并噻吩并嘧啶的前体。所有合成的化合物均通过元素和光谱分析(如 IR、1 H NMR 和13 C NMR)进行表征。此外,还测试了大多数合成化合物对五种真菌菌株的抗真菌活性。此外,化合物3a中,5,6筛选了图1 、图8和图22对HEPG-2和MCF-7细胞系的抗癌活性。
更新日期:2021-05-30
中文翻译:

一些新型噻吩并吡啶并[3',2':4,5]噻吩并[3,2-d]嘧啶和相关杂环的合成、表征和生物活性
合成 3-Cyano-5-ethoxycarbonyl-6-methyl-4-(2'-thienyl)-pyridine-2(1 H )-thion ( 1 ) 并与氯乙酰胺或氯乙腈反应生成 3-amino-5-ethoxycarbonyl -6-甲基-4(2'-噻吩基)-噻吩并[2,3 - b ]吡啶-2-甲酰胺3a或其2-腈类似物3b。的环缩合3A与原甲酸三乙酯中产生的相应pyridothienopyrimidineone 4,其上用磷酰氯中加热得到4-氯嘧啶衍生物5。化合物5作为关键中间体用于合成化合物6 , 9 ,10,11,和12一旦与亲核一些试剂如硫脲处理,5-苯基小号三唑-3(1 ħ) -硫酮,哌啶,吗啉,或水合肼,分别。吡啶并噻吩并嘧啶硫酮6与N- (4-甲苯基)-2-氯乙酰胺或溴乙酸乙酯反应得到相应的S-取代的甲基硫基嘧啶7或8。3b与原甲酸三乙酯缩合得到偶氮甲碱衍生物13, 与水合肼反应生成乙基 3-amino-3,4-dihydro-4-imino-7-methyl-9-(2'-thienyl)pyrido[3',2':4,5]thieno[ 3,2 - d ]嘧啶-8-羧酸酯( 14 )。化合物12和14用作合成其他新的噻吩基吡啶并噻吩并嘧啶以及异构噻吩基-s-三唑并吡啶并噻吩并嘧啶的前体。所有合成的化合物均通过元素和光谱分析(如 IR、1 H NMR 和13 C NMR)进行表征。此外,还测试了大多数合成化合物对五种真菌菌株的抗真菌活性。此外,化合物3a中,5,6筛选了图1 、图8和图22对HEPG-2和MCF-7细胞系的抗癌活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号