当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New approach to consecutive CO oxidation and CO2 chemisorption using Li2CuO2 ceramics modified with Na- and K-molten salts
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2021-5-11 , DOI: 10.1039/d1re00087j Susana Hernández-Castillo 1, 2, 3, 4, 5 , Héctor Martínez-Hernández 6, 7, 8, 9, 10 , J. Arturo Mendoza-Nieto 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2021-5-11 , DOI: 10.1039/d1re00087j Susana Hernández-Castillo 1, 2, 3, 4, 5 , Héctor Martínez-Hernández 6, 7, 8, 9, 10 , J. Arturo Mendoza-Nieto 1, 2, 3, 4, 5
Affiliation
|
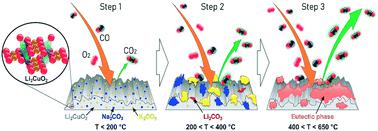
To analyze for the first time the effect of alkali carbonate addition to lithium cuprate during the consecutive process of carbon monoxide (CO) oxidation and subsequent carbon dioxide (CO2) capture, a series of X-containing Li2CuO2 (X = Na and/or K) materials were prepared by mechanically adding different mixtures of sodium and potassium carbonates to lithium cuprate. According to results, the presence of carbonate allowed the improvement of both the CO conversion and the CO2 chemisorption in a moderate temperature range, between 400 and 650 °C. According to the reaction mechanism proposed in this work, this enhancement was produced due to the formation of a new phase between the mechanically added Na and K carbonates and the Li carbonate produced during the CO2 capture on the ceramic surface. The composition of this phase changes depending on the temperature used and the amount of lithium carbonate formed on the surface. Once the newly formed carbonate phase melts, the diffusion of both reactants (CO and O2) is enhanced towards the bulk material, promoting the oxidation of CO and later CO2 capture. Another benefit was detected on the ratio between CO2 captured and released. According to this parameter, the samples modified with a single carbonate, Na- or K–Li2CuO2 samples, showed a high tendency to capture the CO2 formed during the CO oxidation, allowing the simultaneous elimination of two toxic and harmful gases, CO and CO2. The results are promising, considering that at an industrial level, two different materials are required for this process: a heterogeneous catalyst followed by a chemical adsorbent, which can be replaced with a single modified-Li2CuO2 material studied in this work.
中文翻译:

使用Na和K熔融盐改性的Li2CuO2陶瓷进行连续CO氧化和CO2化学吸附的新方法
为了首次分析在连续的一氧化碳(CO)氧化和随后的二氧化碳(CO 2)捕获过程中向碳酸铜盐中添加碱金属碳酸盐的效果,一系列含X的Li 2 CuO 2(X = Na和/或K)材料是通过将碳酸钠和碳酸钾的不同混合物机械添加到铜酸锂中制备的。根据结果,碳酸盐的存在使CO转化率和CO 2均得到改善。在400至650°C的中等温度范围内进行化学吸附。根据这项工作提出的反应机理,这种增强是由于在机械添加的碳酸钠和碳酸钾与在陶瓷表面捕获CO 2期间产生的碳酸锂之间形成了新的相而产生的。该相的组成根据使用的温度和在表面上形成的碳酸锂的量而变化。一旦新形成的碳酸盐相融化,两种反应物(CO和O 2)向主体材料的扩散就会增强,从而促进CO的氧化并随后捕获CO 2。在CO 2之间的比率上发现了另一个好处捕获并释放。根据此参数,用单一碳酸盐Na-或K–Li 2 CuO 2样品改性的样品表现出很强的捕获CO氧化过程中形成的CO 2的趋势,从而可以同时消除两种有毒有害气体, CO和CO 2。考虑到在工业水平上,此过程需要两种不同的材料,因此该结果是令人鼓舞的:一种均相催化剂,然后是一种化学吸附剂,可以用本研究中研究的单一改性Li 2 CuO 2材料代替。
更新日期:2021-05-26
中文翻译:

使用Na和K熔融盐改性的Li2CuO2陶瓷进行连续CO氧化和CO2化学吸附的新方法
为了首次分析在连续的一氧化碳(CO)氧化和随后的二氧化碳(CO 2)捕获过程中向碳酸铜盐中添加碱金属碳酸盐的效果,一系列含X的Li 2 CuO 2(X = Na和/或K)材料是通过将碳酸钠和碳酸钾的不同混合物机械添加到铜酸锂中制备的。根据结果,碳酸盐的存在使CO转化率和CO 2均得到改善。在400至650°C的中等温度范围内进行化学吸附。根据这项工作提出的反应机理,这种增强是由于在机械添加的碳酸钠和碳酸钾与在陶瓷表面捕获CO 2期间产生的碳酸锂之间形成了新的相而产生的。该相的组成根据使用的温度和在表面上形成的碳酸锂的量而变化。一旦新形成的碳酸盐相融化,两种反应物(CO和O 2)向主体材料的扩散就会增强,从而促进CO的氧化并随后捕获CO 2。在CO 2之间的比率上发现了另一个好处捕获并释放。根据此参数,用单一碳酸盐Na-或K–Li 2 CuO 2样品改性的样品表现出很强的捕获CO氧化过程中形成的CO 2的趋势,从而可以同时消除两种有毒有害气体, CO和CO 2。考虑到在工业水平上,此过程需要两种不同的材料,因此该结果是令人鼓舞的:一种均相催化剂,然后是一种化学吸附剂,可以用本研究中研究的单一改性Li 2 CuO 2材料代替。



























 京公网安备 11010802027423号
京公网安备 11010802027423号