当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cadmium(II) Removal by Mackinawite under Anoxic Conditions
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-05-25 , DOI: 10.1021/acsearthspacechem.0c00276 Sung Pil Hyun 1, 2 , Bo-a Kim 1 , Sangbo Son 3 , Kideok D. Kwon 3 , Eungyeong Kim 1 , Kim F. Hayes 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-05-25 , DOI: 10.1021/acsearthspacechem.0c00276 Sung Pil Hyun 1, 2 , Bo-a Kim 1 , Sangbo Son 3 , Kideok D. Kwon 3 , Eungyeong Kim 1 , Kim F. Hayes 2
Affiliation
|
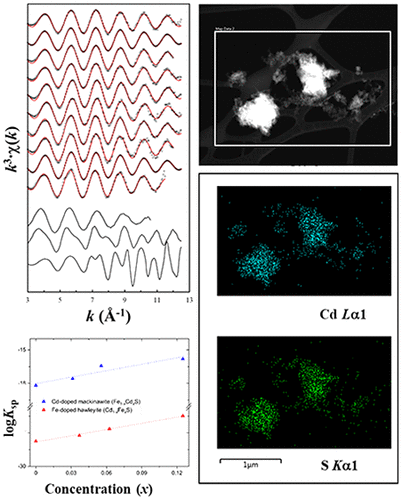
Ferrous monosulfide mackinawite (FeS) forming under iron-rich sulfate-reducing conditions is an effective scavenger of heavy metals and metalloids. In this work, we studied the mechanisms of cadmium(II) [Cd(II)] (a toxic chalcophile metal generally considered redox-inactive) reactions with FeS under an anoxic condition over ranges of geochemical variables. The batch uptake experiments show that dissolved Cd(II) is almost completely removed from the FeS suspensions without a distinct pH or ionic strength dependency under all the experimental conditions studied. The batch uptake results do not support (Cd,Fe)S solid-solution formation. Instead, identification of the solid-phase reaction products by a suite of surface chemical probes suggests that the major Cd reaction product is CdS, with minor amounts of metallic Cd (Cd0) and surface-complexed Cd(II). Density functional theory calculations support the favorability of pure CdS formation compared to Fe-doped CdS or Cd-doped FeS. This study shows that sulfide precipitation largely controls the mobility of Cd, similar to previously reported Hg and As, implicating the presence of dissolved or solid-phase sulfides to be a key parameter in sulfate-reducing natural and engineered environments.
中文翻译:

Mackinawite 在缺氧条件下去除镉 (II)
在富含铁的硫酸盐还原条件下形成的单硫化亚铁麦基诺矿 (FeS) 是重金属和准金属的有效清除剂。在这项工作中,我们研究了镉 (II) [Cd(II)](一种有毒的亲硫金属,通常被认为不具有氧化还原活性)在地球化学变量范围内的缺氧条件下与 FeS 反应的机制。批量吸收实验表明,在所有研究的实验条件下,溶解的 Cd(II) 几乎完全从 FeS 悬浮液中去除,没有明显的 pH 值或离子强度依赖性。批次吸收结果不支持 (Cd,Fe)S 固溶体形成。相反,通过一套表面化学探针对固相反应产物的鉴定表明主要的 Cd 反应产物是 CdS,还有少量的金属 Cd(Cd 0) 和表面复合的 Cd(II)。与 Fe 掺杂的 CdS 或 Cd 掺杂的 FeS 相比,密度泛函理论计算支持纯 CdS 形成的有利性。这项研究表明,硫化物沉淀在很大程度上控制了 Cd 的迁移率,类似于先前报道的 Hg 和 As,这意味着溶解或固相硫化物的存在是硫酸盐还原自然和工程环境中的关键参数。
更新日期:2021-06-17
中文翻译:

Mackinawite 在缺氧条件下去除镉 (II)
在富含铁的硫酸盐还原条件下形成的单硫化亚铁麦基诺矿 (FeS) 是重金属和准金属的有效清除剂。在这项工作中,我们研究了镉 (II) [Cd(II)](一种有毒的亲硫金属,通常被认为不具有氧化还原活性)在地球化学变量范围内的缺氧条件下与 FeS 反应的机制。批量吸收实验表明,在所有研究的实验条件下,溶解的 Cd(II) 几乎完全从 FeS 悬浮液中去除,没有明显的 pH 值或离子强度依赖性。批次吸收结果不支持 (Cd,Fe)S 固溶体形成。相反,通过一套表面化学探针对固相反应产物的鉴定表明主要的 Cd 反应产物是 CdS,还有少量的金属 Cd(Cd 0) 和表面复合的 Cd(II)。与 Fe 掺杂的 CdS 或 Cd 掺杂的 FeS 相比,密度泛函理论计算支持纯 CdS 形成的有利性。这项研究表明,硫化物沉淀在很大程度上控制了 Cd 的迁移率,类似于先前报道的 Hg 和 As,这意味着溶解或固相硫化物的存在是硫酸盐还原自然和工程环境中的关键参数。









































 京公网安备 11010802027423号
京公网安备 11010802027423号