当前位置:
X-MOL 学术
›
Mol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cysteine Modification by Ebselen Reduces the Stability and Cellular Levels of 14-3-3 Proteins
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2021-08-01 , DOI: 10.1124/molpharm.120.000184 Kai Waløen 1 , Kunwar Jung-Kc 1 , Elisa D Vecchia 1 , Sunil Pandey 1 , Norbert Gasparik 1 , Anne Døskeland 1 , Sudarshan Patil 1 , Rune Kleppe 1 , Jozef Hritz 1 , William H J Norton 1 , Aurora Martinez 2 , Jan Haavik 2
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2021-08-01 , DOI: 10.1124/molpharm.120.000184 Kai Waløen 1 , Kunwar Jung-Kc 1 , Elisa D Vecchia 1 , Sunil Pandey 1 , Norbert Gasparik 1 , Anne Døskeland 1 , Sudarshan Patil 1 , Rune Kleppe 1 , Jozef Hritz 1 , William H J Norton 1 , Aurora Martinez 2 , Jan Haavik 2
Affiliation
|
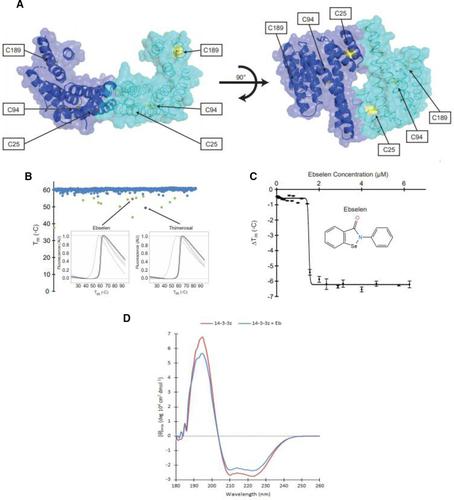
The 14-3-3 proteins constitute a family of adaptor proteins with many binding partners and biological functions, and they are considered promising drug targets in cancer and neuropsychiatry. By screening 1280 small-molecule drugs using differential scanning fluorimetry (DSF), we found 15 compounds that decreased the thermal stability of 14-3-3ζ. Among these compounds, ebselen was identified as a covalent, destabilizing ligand of 14-3-3 isoforms ζ, ε, γ, and η. Ebselen bonding decreased 14-3-3ζ binding to its partner Ser19-phosphorylated tyrosine hydroxylase. Characterization of site-directed mutants at cysteine residues in 14-3-3ζ (C25, C94, and C189) by DSF and mass spectroscopy revealed covalent modification by ebselen of all cysteines through a selenylsulfide bond. C25 appeared to be the preferential site of ebselen interaction in vitro, whereas modification of C94 was the main determinant for protein destabilization. At therapeutically relevant concentrations, ebselen and ebselen oxide caused decreased 14-3-3 levels in SH-SY5Y cells, accompanied with an increased degradation, most probably by the ubiquitin-dependent proteasome pathway. Moreover, ebselen-treated zebrafish displayed decreased brain 14-3-3 content, a freezing phenotype, and reduced mobility, resembling the effects of lithium, consistent with its proposed action as a safer lithium-mimetic drug. Ebselen has recently emerged as a promising drug candidate in several medical areas, such as cancer, neuropsychiatric disorders, and infectious diseases, including coronavirus disease 2019. Its pleiotropic actions are attributed to antioxidant effects and formation of selenosulfides with critical cysteine residues in proteins. Our work indicates that a destabilization of 14-3-3 may affect the protein interaction networks of this protein family, contributing to the therapeutic potential of ebselen.
中文翻译:

Ebselen 的半胱氨酸修饰降低了 14-3-3 蛋白质的稳定性和细胞水平
14-3-3 蛋白构成了一个具有许多结合伙伴和生物学功能的衔接蛋白家族,它们被认为是癌症和神经精神病学中很有前景的药物靶点。通过使用差示扫描荧光法 (DSF) 筛选 1280 种小分子药物,我们发现了 15 种降低 14-3-3 ζ热稳定性的化合物。在这些化合物中,依布硒啉被鉴定为 14-3-3 亚型ζ、ε、γ和η的共价不稳定配体。Ebselen 键合降低了 14-3-3 ζ与其伴侣 Ser19 磷酸化酪氨酸羟化酶的结合。14-3-3 ζ 中半胱氨酸残基定点突变体的表征(C25、C94 和 C189)通过 DSF 和质谱分析显示,依布硒啉通过硒硫键对所有半胱氨酸进行共价修饰。C25 在体外似乎是依布硒啉相互作用的优先位点,而 C94 的修饰是蛋白质不稳定的主要决定因素。在治疗相关浓度下,依布硒啉和依布硒啉氧化物导致 SH-SY5Y 细胞中 14-3-3 水平降低,同时降解增加,最有可能是泛素依赖性蛋白酶体途径。此外,依布硒啉处理的斑马鱼表现出脑 14-3-3 含量降低、冻结表型和活动性降低,类似于锂的作用,与其作为更安全的锂模拟药物的建议作用一致。Ebselen 最近已成为多个医学领域的有前途的候选药物,例如癌症、神经精神疾病和传染病,包括 2019 年冠状病毒病。其多效作用归因于抗氧化作用和蛋白质中具有关键半胱氨酸残基的硒硫化物的形成。我们的工作表明 14-3-3 的不稳定可能会影响该蛋白质家族的蛋白质相互作用网络,从而促进依布硒啉的治疗潜力。
更新日期:2021-08-31
中文翻译:

Ebselen 的半胱氨酸修饰降低了 14-3-3 蛋白质的稳定性和细胞水平
14-3-3 蛋白构成了一个具有许多结合伙伴和生物学功能的衔接蛋白家族,它们被认为是癌症和神经精神病学中很有前景的药物靶点。通过使用差示扫描荧光法 (DSF) 筛选 1280 种小分子药物,我们发现了 15 种降低 14-3-3 ζ热稳定性的化合物。在这些化合物中,依布硒啉被鉴定为 14-3-3 亚型ζ、ε、γ和η的共价不稳定配体。Ebselen 键合降低了 14-3-3 ζ与其伴侣 Ser19 磷酸化酪氨酸羟化酶的结合。14-3-3 ζ 中半胱氨酸残基定点突变体的表征(C25、C94 和 C189)通过 DSF 和质谱分析显示,依布硒啉通过硒硫键对所有半胱氨酸进行共价修饰。C25 在体外似乎是依布硒啉相互作用的优先位点,而 C94 的修饰是蛋白质不稳定的主要决定因素。在治疗相关浓度下,依布硒啉和依布硒啉氧化物导致 SH-SY5Y 细胞中 14-3-3 水平降低,同时降解增加,最有可能是泛素依赖性蛋白酶体途径。此外,依布硒啉处理的斑马鱼表现出脑 14-3-3 含量降低、冻结表型和活动性降低,类似于锂的作用,与其作为更安全的锂模拟药物的建议作用一致。Ebselen 最近已成为多个医学领域的有前途的候选药物,例如癌症、神经精神疾病和传染病,包括 2019 年冠状病毒病。其多效作用归因于抗氧化作用和蛋白质中具有关键半胱氨酸残基的硒硫化物的形成。我们的工作表明 14-3-3 的不稳定可能会影响该蛋白质家族的蛋白质相互作用网络,从而促进依布硒啉的治疗潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号