当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A division of labor between two biotin protein ligase homologs
Molecular Microbiology ( IF 2.6 ) Pub Date : 2021-05-24 , DOI: 10.1111/mmi.14761 Xuejiao Song 1 , Sarah K Henke 2 , John E Cronan 1, 2
Molecular Microbiology ( IF 2.6 ) Pub Date : 2021-05-24 , DOI: 10.1111/mmi.14761 Xuejiao Song 1 , Sarah K Henke 2 , John E Cronan 1, 2
Affiliation
|
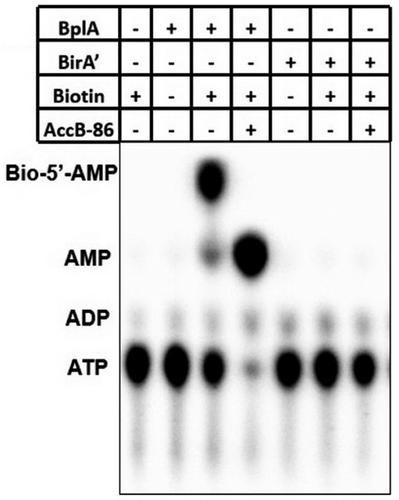
Group I biotin protein ligases (BPLs) catalyze the covalent attachment of biotin to its cognate acceptor proteins. In contrast, Group II BPLs have an additional N-terminal DNA-binding domain and function not only in biotinylation but also in transcriptional regulation of genes of biotin biosynthesis and transport. Most bacteria contain only a single biotin protein ligase, whereas Clostridium acetobutylicum contains two biotin protein ligase homologs: BplA and BirA′. Sequence alignments showed that BplA is a typical group I BPL, whereas BirA′ lacked the C-terminal domain conserved throughout extant BPL proteins. This raised the questions of why two BPL homologs are needed and why the apparently defective BirA′ has been retained. We have used in vivo and in vitro assays to show that BplA is a functional BPL whereas BirA′ acts as a biotin sensor involved in transcriptional regulation of biotin transport. We also successfully converted BirA′ into a functional biotin protein ligase with regulatory activity by fusing it to the C-terminal domain from BplA. Finally, we provide evidence that BplA and BirA′ interact in vivo.
中文翻译:

两种生物素蛋白连接酶同源物之间的分工
I 组生物素蛋白连接酶 (BPL) 催化生物素与其同源受体蛋白的共价连接。相比之下,II 组 BPL 有一个额外的 N 端 DNA 结合结构域,不仅在生物素化中起作用,而且在生物素生物合成和转运基因的转录调节中起作用。大多数细菌仅含有一种生物素蛋白连接酶,而丙酮丁醇梭菌含有两种生物素蛋白连接酶同源物:BplA 和 BirA'。序列比对显示 BplA 是典型的 I 组 BPL,而 BirA' 缺乏在现存 BPL 蛋白中保守的 C 端结构域。这就提出了为什么需要两个 BPL 同源物以及为什么保留了明显有缺陷的 BirA' 的问题。我们已经使用体内和体外测定表明 BplA 是一种功能性 BPL,而 BirA' 充当生物素传感器,参与生物素转运的转录调节。我们还通过将 BirA' 与 BplA 的 C 端结构域融合,成功地将 BirA' 转化为具有调节活性的功能性生物素蛋白连接酶。最后,我们提供了 BplA 和 BirA' 在体内相互作用的证据。
更新日期:2021-05-24
中文翻译:

两种生物素蛋白连接酶同源物之间的分工
I 组生物素蛋白连接酶 (BPL) 催化生物素与其同源受体蛋白的共价连接。相比之下,II 组 BPL 有一个额外的 N 端 DNA 结合结构域,不仅在生物素化中起作用,而且在生物素生物合成和转运基因的转录调节中起作用。大多数细菌仅含有一种生物素蛋白连接酶,而丙酮丁醇梭菌含有两种生物素蛋白连接酶同源物:BplA 和 BirA'。序列比对显示 BplA 是典型的 I 组 BPL,而 BirA' 缺乏在现存 BPL 蛋白中保守的 C 端结构域。这就提出了为什么需要两个 BPL 同源物以及为什么保留了明显有缺陷的 BirA' 的问题。我们已经使用体内和体外测定表明 BplA 是一种功能性 BPL,而 BirA' 充当生物素传感器,参与生物素转运的转录调节。我们还通过将 BirA' 与 BplA 的 C 端结构域融合,成功地将 BirA' 转化为具有调节活性的功能性生物素蛋白连接酶。最后,我们提供了 BplA 和 BirA' 在体内相互作用的证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号