Journal of Structural Biology ( IF 3 ) Pub Date : 2021-05-24 , DOI: 10.1016/j.jsb.2021.107748 Keito Hiragi 1 , Kazuya Nishio 1 , Shu Moriyama 1 , Tasuku Hamaguchi 2 , Akira Mizoguchi 3 , Koji Yonekura 4 , Kazutoshi Tani 3 , Tsunehiro Mizushima 1
|
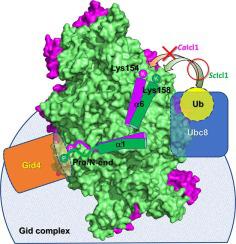
In Saccharomyces cerevisiae, the glyoxylate cycle is controlled through the posttranslational regulation of its component enzymes, such as isocitrate lyase (ICL), which catalyzes the first unique step of the cycle. The ICL of S. cerevisiae (ScIcl1) is tagged for proteasomal degradation through ubiquitination by a multisubunit ubiquitin ligase (the glucose-induced degradation-deficient (GID) complex), whereas that of the pathogenic yeast Candida albicans (CaIcl1) escapes this process. However, the reason for the ubiquitin targeting specificity of the GID complex for ScIcl1 and not for CaIcl1 is unclear. To gain some insight into this, in this study, the crystal structures of apo ScIcl1 and CaIcl1 in complex with formate and the cryogenic electron microscopy structure of apo CaIcl1 were determined at a resolution of 2.3, 2.7, and 2.6 Å, respectively. A comparison of the various structures suggests that the orientation of N-terminal helix α1 in S. cerevisiae is likely key to repositioning of ubiquitination sites and contributes to the distinction found in C. albicans ubiquitin evasion mechanism. This finding gives us a better understanding of the molecular mechanism of ubiquitin-dependent ScIcl1 degradation and could serve as a theoretical basis for the research and development of anti-C. albicans drugs based on the concept of CaIcl1 ubiquitination.
中文翻译:

泛素连接酶对酿酒酵母异柠檬酸裂合酶而非白色念珠菌异柠檬酸裂合酶的靶向特异性的结构见解
在酿酒酵母中,乙醛酸循环通过其组分酶的翻译后调节来控制,例如异柠檬酸裂解酶 (ICL),它催化循环的第一个独特步骤。S的 ICL 。 酿酒酵母( Sc Icl1) 被标记为通过多亚基泛素连接酶(葡萄糖诱导的降解缺陷 (GID) 复合物)泛素化进行蛋白酶体降解,而致病性酵母白色念珠菌( Ca Icl1) 则逃脱了这一过程。然而,GID复合物对Sc Icl1而不是Ca的泛素靶向特异性的原因Icl1 不清楚。为了深入了解这一点,在本研究中,apo Sc Icl1 和Ca Icl1 与甲酸盐复合的晶体结构和 apo Ca Icl1 的低温电子显微镜结构分别以 2.3、2.7 和 2.6 Å 的分辨率测定. 各种结构的比较表明S中 N 端螺旋 α1 的方向。 酿酒酵母可能是重新定位泛素化位点的关键,并有助于在C中发现的区别。白化病患者泛素逃避机制。这一发现使我们对泛素依赖性Sc的分子机制有了更好的理解Icl1 降解,可作为抗C研究开发的理论基础。基于Ca Icl1 泛素化概念的白色念珠菌药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号