当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The impact of oncogenic mutations of the viral Src kinase on the structure and stability of the SH3 domain
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-05-19 , DOI: 10.1107/s2059798321004344 M Carmen Salinas-Garcia 1 , Marina Plaza-Garrido 1 , Ana Camara-Artigas 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-05-19 , DOI: 10.1107/s2059798321004344 M Carmen Salinas-Garcia 1 , Marina Plaza-Garrido 1 , Ana Camara-Artigas 1
Affiliation
|
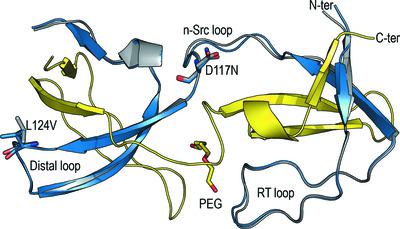
Src kinase belongs to the family of Src-related nonreceptor tyrosine kinases. Because of its physiological role in cell growth and proliferation, its activity is strictly controlled by several mechanisms. Nevertheless, in viral Src kinase (v-Src) some of these mechanisms fail, and its uncontrolled activity is responsible for the occurrence of cancer. Here, the crystal structures of three SH3-domain mutants of v-Src were determined to unveil the effects of these oncogenic mutations in this regulatory domain. Mutations in the n-Src and distal loops have a low impact on the overall structure of the domain and its capacity to form intertwined dimers. However, mutations in the RT loop compromise the stability of the domain and make the protein very prone to aggregation. Additionally, these mutations prevent the formation of intertwined dimers. The results show a synergistic effect between mutations in the RT loop and those in the n-Src and distal loops. Analysis of the structures of the v-Src SH3-domain mutants and the closed inactive conformation of cellular Src kinase (c-Src) point to a loss of the interactions that are required to establish the compact inactive form of the kinase. Nevertheless, an analysis of structures of the c-Src SH3 domain complexed with class I and II peptides points to minor changes in the interactions between the v-Src SH3 domain and these peptides. In this way, the structures reported here indicate that mutations in the RT loop might impair the kinase regulation mechanism without affecting the recognition of short proline-rich motifs in the target proteins of the kinase, thus explaining the oncogenic behaviour of the protein.
中文翻译:

病毒Src激酶致癌突变对SH3结构域结构和稳定性的影响
Src 激酶属于 Src 相关非受体酪氨酸激酶家族。由于其在细胞生长和增殖中的生理作用,其活性受到多种机制的严格控制。然而,在病毒 Src 激酶 (v-Src) 中,其中一些机制失败,其不受控制的活性导致癌症的发生。在这里,确定了 v-Src 的三个 SH3 结构域突变体的晶体结构,以揭示这些致癌突变在该调节结构域中的影响。n-Src 和远端环中的突变对该结构域的整体结构及其形成交织二聚体的能力影响较小。然而,RT 环中的突变会损害结构域的稳定性,并使蛋白质非常容易聚集。此外,这些突变阻止了相互缠绕的二聚体的形成。结果显示 RT 环中的突变与 n-Src 和远端环中的突变之间存在协同效应。对 v-Src SH3 结构域突变体和细胞 Src 激酶 (c-Src) 的闭合非活性构象的结构分析表明,建立激酶的紧凑非活性形式所需的相互作用丧失。然而,对与 I 类和 II 类肽复合的 c-Src SH3 结构域的结构分析表明,v-Src SH3 结构域与这些肽之间的相互作用发生了微小变化。通过这种方式,本文报道的结构表明,RT环中的突变可能会损害激酶调节机制,而不影响激酶靶蛋白中富含脯氨酸的短基序的识别,从而解释了该蛋白的致癌行为。
更新日期:2021-06-02
中文翻译:

病毒Src激酶致癌突变对SH3结构域结构和稳定性的影响
Src 激酶属于 Src 相关非受体酪氨酸激酶家族。由于其在细胞生长和增殖中的生理作用,其活性受到多种机制的严格控制。然而,在病毒 Src 激酶 (v-Src) 中,其中一些机制失败,其不受控制的活性导致癌症的发生。在这里,确定了 v-Src 的三个 SH3 结构域突变体的晶体结构,以揭示这些致癌突变在该调节结构域中的影响。n-Src 和远端环中的突变对该结构域的整体结构及其形成交织二聚体的能力影响较小。然而,RT 环中的突变会损害结构域的稳定性,并使蛋白质非常容易聚集。此外,这些突变阻止了相互缠绕的二聚体的形成。结果显示 RT 环中的突变与 n-Src 和远端环中的突变之间存在协同效应。对 v-Src SH3 结构域突变体和细胞 Src 激酶 (c-Src) 的闭合非活性构象的结构分析表明,建立激酶的紧凑非活性形式所需的相互作用丧失。然而,对与 I 类和 II 类肽复合的 c-Src SH3 结构域的结构分析表明,v-Src SH3 结构域与这些肽之间的相互作用发生了微小变化。通过这种方式,本文报道的结构表明,RT环中的突变可能会损害激酶调节机制,而不影响激酶靶蛋白中富含脯氨酸的短基序的识别,从而解释了该蛋白的致癌行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号