当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of human factor VIIa–soluble tissue factor with calcium, magnesium and rubidium
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-05-24 , DOI: 10.1107/s2059798321003922 Kanagasabai Vadivel 1 , Amy E Schmidt 2 , Duilio Cascio 3 , Kaillathe Padmanabhan 4 , Sriram Krishnaswamy 5 , Hans Brandstetter 6 , S Paul Bajaj 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-05-24 , DOI: 10.1107/s2059798321003922 Kanagasabai Vadivel 1 , Amy E Schmidt 2 , Duilio Cascio 3 , Kaillathe Padmanabhan 4 , Sriram Krishnaswamy 5 , Hans Brandstetter 6 , S Paul Bajaj 1
Affiliation
|
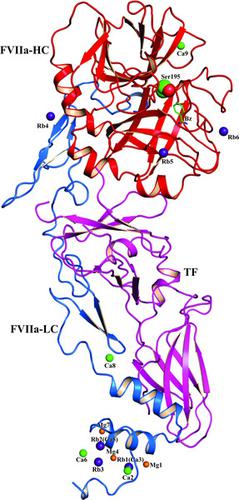
Coagulation factor VIIa (FVIIa) consists of a γ-carboxyglutamic acid (GLA) domain, two epidermal growth factor-like (EGF) domains and a protease domain. FVIIa binds three Mg2+ ions and four Ca2+ ions in the GLA domain, one Ca2+ ion in the EGF1 domain and one Ca2+ ion in the protease domain. Further, FVIIa contains an Na+ site in the protease domain. Since Na+ and water share the same number of electrons, Na+ sites in proteins are difficult to distinguish from waters in X-ray structures. Here, to verify the Na+ site in FVIIa, the structure of the FVIIa–soluble tissue factor (TF) complex was solved at 1.8 Å resolution containing Mg2+, Ca2+ and Rb+ ions. In this structure, Rb+ replaced two Ca2+ sites in the GLA domain and occupied three non-metal sites in the protease domain. However, Rb+ was not detected at the expected Na+ site. In kinetic experiments, Na+ increased the amidolytic activity of FVIIa towards the synthetic substrate S-2288 (H-d-Ile-Pro-Arg-p-nitroanilide) by ∼20-fold; however, in the presence of Ca2+, Na+ had a negligible effect. Ca2+ increased the hydrolytic activity of FVIIa towards S-2288 by ∼60-fold in the absence of Na+ and by ∼82-fold in the presence of Na+. In molecular-dynamics simulations, Na+ stabilized the two Na+-binding loops (the 184-loop and 220-loop) and the TF-binding region spanning residues 163–180. Ca2+ stabilized the Ca2+-binding loop (the 70-loop) and Na+-binding loops but not the TF-binding region. Na+ and Ca2+ together stabilized both the Na+-binding and Ca2+-binding loops and the TF-binding region. Previously, Rb+ has been used to define the Na+ site in thrombin; however, it was unsuccessful in detecting the Na+ site in FVIIa. A conceivable explanation for this observation is provided.
中文翻译:

含钙、镁和铷的人因子 VIIa-可溶性组织因子的结构
凝血因子 VIIa (FVIIa) 由一个 γ-羧基谷氨酸 (GLA) 结构域、两个表皮生长因子样 (EGF) 结构域和一个蛋白酶结构域组成。FVIIa在GLA结构域中结合3个Mg 2+离子和4个Ca 2+离子,在EGF1结构域中结合1个Ca 2+离子,在蛋白酶结构域中结合1个Ca 2+离子。此外,FVIIa在蛋白酶结构域中含有Na +位点。由于Na +和水共享相同数量的电子,因此蛋白质中的Na +位点很难与X 射线结构中的水区分开。在这里,为了验证 FVIIa 中的 Na +位点,以 1.8 Å 分辨率解析了包含 Mg 2+、Ca 2+和 Rb +离子的 FVIIa-可溶性组织因子 (TF) 复合物的结构。在该结构中,Rb +取代了GLA结构域中的两个Ca 2+位点,并占据了蛋白酶结构域中的三个非金属位点。然而,在预期的Na +位点没有检测到Rb +。在动力学实验中,Na +使 FVIIa 对合成底物 S-2288 (H- d -Ile-Pro-Arg -p-硝基苯胺)的酰胺分解活性增加约 20 倍;然而,在Ca 2+存在的情况下,Na + 的影响可以忽略不计。Ca 2+使 FVIIa 对 S-2288 的水解活性在不存在 Na +的情况下增加约 60 倍,在存在 Na +的情况下增加约 82 倍。在分子动力学模拟中,Na +稳定了两个 Na +结合环(184 环和 220 环)和跨越残基 163-180 的 TF 结合区域。Ca 2+稳定Ca 2+结合环(70 环)和Na +结合环,但不稳定TF 结合区域。Na +和Ca 2+一起稳定Na +结合环和Ca 2+结合环以及TF 结合区域。此前,Rb +已被用来定义凝血酶中的Na +位点;然而,未能成功检测到FVIIa中的Na +位点。对此观察结果提供了一个可以想象的解释。
更新日期:2021-06-02
中文翻译:

含钙、镁和铷的人因子 VIIa-可溶性组织因子的结构
凝血因子 VIIa (FVIIa) 由一个 γ-羧基谷氨酸 (GLA) 结构域、两个表皮生长因子样 (EGF) 结构域和一个蛋白酶结构域组成。FVIIa在GLA结构域中结合3个Mg 2+离子和4个Ca 2+离子,在EGF1结构域中结合1个Ca 2+离子,在蛋白酶结构域中结合1个Ca 2+离子。此外,FVIIa在蛋白酶结构域中含有Na +位点。由于Na +和水共享相同数量的电子,因此蛋白质中的Na +位点很难与X 射线结构中的水区分开。在这里,为了验证 FVIIa 中的 Na +位点,以 1.8 Å 分辨率解析了包含 Mg 2+、Ca 2+和 Rb +离子的 FVIIa-可溶性组织因子 (TF) 复合物的结构。在该结构中,Rb +取代了GLA结构域中的两个Ca 2+位点,并占据了蛋白酶结构域中的三个非金属位点。然而,在预期的Na +位点没有检测到Rb +。在动力学实验中,Na +使 FVIIa 对合成底物 S-2288 (H- d -Ile-Pro-Arg -p-硝基苯胺)的酰胺分解活性增加约 20 倍;然而,在Ca 2+存在的情况下,Na + 的影响可以忽略不计。Ca 2+使 FVIIa 对 S-2288 的水解活性在不存在 Na +的情况下增加约 60 倍,在存在 Na +的情况下增加约 82 倍。在分子动力学模拟中,Na +稳定了两个 Na +结合环(184 环和 220 环)和跨越残基 163-180 的 TF 结合区域。Ca 2+稳定Ca 2+结合环(70 环)和Na +结合环,但不稳定TF 结合区域。Na +和Ca 2+一起稳定Na +结合环和Ca 2+结合环以及TF 结合区域。此前,Rb +已被用来定义凝血酶中的Na +位点;然而,未能成功检测到FVIIa中的Na +位点。对此观察结果提供了一个可以想象的解释。











































 京公网安备 11010802027423号
京公网安备 11010802027423号