Structure ( IF 4.4 ) Pub Date : 2021-05-20 , DOI: 10.1016/j.str.2021.05.003 Sunbin Deng 1 , Leah Gottlieb 1 , Buyan Pan 2 , Julianna Supplee 3 , Xuepeng Wei 4 , E James Petersson 2 , Ronen Marmorstein 5
|
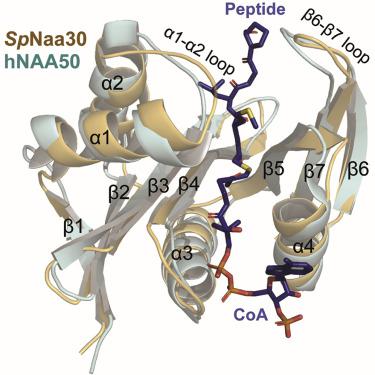
Protein N-terminal acetylation is predominantly a ribosome-associated modification, with NatA-E serving as the major enzymes. NatC is the most unusual of these enzymes, containing one Naa30 catalytic subunit and two auxiliary subunits, Naa35 and Naa38; and substrate selectivity profile that overlaps with NatE. Here, we report the cryoelectron microscopy structure of S. pombe NatC with a NatE/C-type bisubstrate analog and inositol hexaphosphate (IP6), and associated biochemistry studies. We find that the presence of three subunits is a prerequisite for normal NatC acetylation activity in yeast and that IP6 binds tightly to NatC to stabilize the complex. We also describe the molecular basis for IP6-mediated NatC complex stabilization and the overlapping yet distinct substrate profiles of NatC and NatE.
中文翻译:

三元NatC复合物N端乙酰化的分子机制
蛋白质 N 端乙酰化主要是与核糖体相关的修饰,其中 NatA-E 是主要的酶。NatC 是这些酶中最不寻常的,包含一个 Naa30 催化亚基和两个辅助亚基 Naa35 和 Naa38;和与 NatE 重叠的底物选择性分布。在这里,我们报告了具有 NatE/C 型双底物类似物和肌醇六磷酸 (IP 6 ) 的S. pombe NatC的低温电子显微镜结构,以及相关的生物化学研究。我们发现三个亚基的存在是酵母中正常 NatC 乙酰化活性的先决条件,并且 IP 6与 NatC 紧密结合以稳定复合物。我们还描述了 IP 6的分子基础-介导的 NatC 复合物稳定性和 NatC 和 NatE 的重叠但不同的底物谱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号