当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Emerging Role of the Innate Immune Response in Idiosyncratic Drug Reactions
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2021-07-01 , DOI: 10.1124/pharmrev.120.000090 Samantha Christine Sernoskie 1 , Alison Jee 1 , Jack Paul Uetrecht 2
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2021-07-01 , DOI: 10.1124/pharmrev.120.000090 Samantha Christine Sernoskie 1 , Alison Jee 1 , Jack Paul Uetrecht 2
Affiliation
|
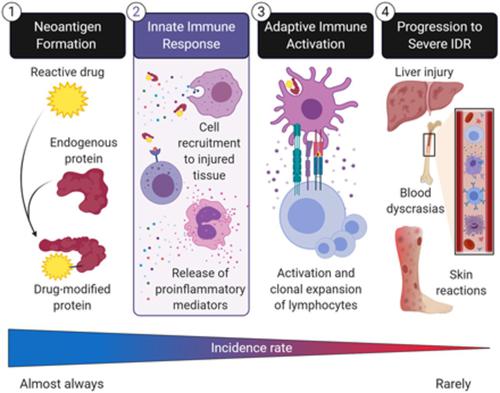
Idiosyncratic drug reactions (IDRs) range from relatively common, mild reactions to rarer, potentially life-threatening adverse effects that pose significant risks to both human health and successful drug discovery. Most frequently, IDRs target the liver, skin, and blood or bone marrow. Clinical data indicate that most IDRs are mediated by an adaptive immune response against drug-modified proteins, formed when chemically reactive species of a drug bind to self-proteins, making them appear foreign to the immune system. Although much emphasis has been placed on characterizing the clinical presentation of IDRs and noting implicated drugs, limited research has focused on the mechanisms preceding the manifestations of these severe responses. Therefore, we propose that to address the knowledge gap between drug administration and onset of a severe IDR, more research is required to understand IDR-initiating mechanisms; namely, the role of the innate immune response. In this review, we outline the immune processes involved from neoantigen formation to the result of the formation of the immunologic synapse and suggest that this framework be applied to IDR research. Using four drugs associated with severe IDRs as examples (amoxicillin, amodiaquine, clozapine, and nevirapine), we also summarize clinical and animal model data that are supportive of an early innate immune response. Finally, we discuss how understanding the early steps in innate immune activation in the development of an adaptive IDR will be fundamental in risk assessment during drug development.
中文翻译:

先天免疫反应在特异药物反应中的新兴作用
特殊药物反应 (IDR) 范围从相对常见的轻微反应到罕见的、可能危及生命的不良反应,这些不良反应对人类健康和成功的药物发现构成重大风险。最常见的是,IDR 靶向肝脏、皮肤、血液或骨髓。临床数据表明,大多数 IDR 是由针对药物修饰蛋白的适应性免疫反应介导的,当药物的化学活性物质与自身蛋白结合时形成,使它们对免疫系统显得陌生。尽管已将重点放在表征 IDR 的临床表现和注意相关药物上,但有限的研究集中在这些严重反应表现之前的机制上。因此,我们建议解决药物管理与严重 IDR 发作之间的知识差距,需要更多的研究来了解 IDR 启动机制;即先天免疫反应的作用。在这篇综述中,我们概述了从新抗原形成到免疫突触形成结果所涉及的免疫过程,并建议将该框架应用于 IDR 研究。以与严重 IDR 相关的四种药物(阿莫西林、阿莫地喹、氯氮平和奈韦拉平)为例,我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。我们概述了从新抗原形成到免疫突触形成结果所涉及的免疫过程,并建议将该框架应用于 IDR 研究。以与严重 IDR 相关的四种药物(阿莫西林、阿莫地喹、氯氮平和奈韦拉平)为例,我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。我们概述了从新抗原形成到免疫突触形成结果所涉及的免疫过程,并建议将该框架应用于 IDR 研究。以与严重 IDR 相关的四种药物(阿莫西林、阿莫地喹、氯氮平和奈韦拉平)为例,我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。和奈韦拉平),我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。和奈韦拉平),我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。
更新日期:2021-05-22
中文翻译:

先天免疫反应在特异药物反应中的新兴作用
特殊药物反应 (IDR) 范围从相对常见的轻微反应到罕见的、可能危及生命的不良反应,这些不良反应对人类健康和成功的药物发现构成重大风险。最常见的是,IDR 靶向肝脏、皮肤、血液或骨髓。临床数据表明,大多数 IDR 是由针对药物修饰蛋白的适应性免疫反应介导的,当药物的化学活性物质与自身蛋白结合时形成,使它们对免疫系统显得陌生。尽管已将重点放在表征 IDR 的临床表现和注意相关药物上,但有限的研究集中在这些严重反应表现之前的机制上。因此,我们建议解决药物管理与严重 IDR 发作之间的知识差距,需要更多的研究来了解 IDR 启动机制;即先天免疫反应的作用。在这篇综述中,我们概述了从新抗原形成到免疫突触形成结果所涉及的免疫过程,并建议将该框架应用于 IDR 研究。以与严重 IDR 相关的四种药物(阿莫西林、阿莫地喹、氯氮平和奈韦拉平)为例,我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。我们概述了从新抗原形成到免疫突触形成结果所涉及的免疫过程,并建议将该框架应用于 IDR 研究。以与严重 IDR 相关的四种药物(阿莫西林、阿莫地喹、氯氮平和奈韦拉平)为例,我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。我们概述了从新抗原形成到免疫突触形成结果所涉及的免疫过程,并建议将该框架应用于 IDR 研究。以与严重 IDR 相关的四种药物(阿莫西林、阿莫地喹、氯氮平和奈韦拉平)为例,我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。和奈韦拉平),我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。和奈韦拉平),我们还总结了支持早期先天免疫反应的临床和动物模型数据。最后,我们讨论了了解适应性 IDR 开发过程中先天免疫激活的早期步骤将如何成为药物开发过程中风险评估的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号