当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel imidazo[2,1-b]thiazole-based anticancer agents as potential focal adhesion kinase inhibitors: Synthesis, in silico and in vitro evaluation
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2021-05-22 , DOI: 10.1111/cbdd.13896 Faika Başoğlu 1, 2 , Nuray Ulusoy-Güzeldemirci 1 , Gülşen Akalın-Çiftçi 3 , Serap Çetinkaya 4 , Abdulilah Ece 5
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2021-05-22 , DOI: 10.1111/cbdd.13896 Faika Başoğlu 1, 2 , Nuray Ulusoy-Güzeldemirci 1 , Gülşen Akalın-Çiftçi 3 , Serap Çetinkaya 4 , Abdulilah Ece 5
Affiliation
|
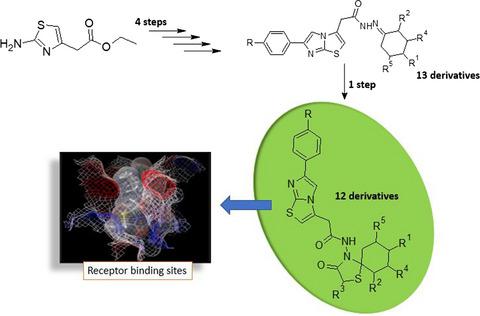
The purpose of this study was to synthesize imidazo[2,1-b]thiazole derivatives, characterize them with spectroscopical techniques and investigate for cytotoxic and apoptotic effects on glioma C6 cancer cell line. The in vitro anticancer activities were also investigated against focal adhesion kinase. Most of the compounds, particularly the derivatives carrying 3-oxo-1-tiya-4-azaspiro[4.5]decane moiety, exhibited higher or comparable activities in comparison with the reference drug, cisplatin. Compounds with methyl, propyl, phenyl moieties at the eighth and second position of the spirothiazolidinone ring showed high FAK inhibitory activities. In addition, molecular docking studies shed light on the binding modes of the synthesized compounds. The critical interactions with amino acid residues located in the active site were revealed. The results obtained from both biological assay data and computational results might provide insight into developing new inhibitors against focal adhesion kinase.
中文翻译:

作为潜在粘着斑激酶抑制剂的新型咪唑并[2,1-b]噻唑类抗癌剂:合成、计算机和体外评估
本研究的目的是合成咪唑[2,1- b]噻唑衍生物,用光谱技术对它们进行表征,并研究对神经胶质瘤 C6 癌细胞系的细胞毒性和凋亡作用。还研究了针对粘着斑激酶的体外抗癌活性。大多数化合物,特别是带有 3-oxo-1-tiya-4-azaspiro[4.5]decane 部分的衍生物,与参考药物顺铂相比,表现出更高或相当的活性。在螺噻唑烷酮环的第八位和第二位具有甲基、丙基、苯基部分的化合物显示出高的 FAK 抑制活性。此外,分子对接研究揭示了合成化合物的结合模式。揭示了与位于活性位点的氨基酸残基的关键相互作用。
更新日期:2021-07-14
中文翻译:

作为潜在粘着斑激酶抑制剂的新型咪唑并[2,1-b]噻唑类抗癌剂:合成、计算机和体外评估
本研究的目的是合成咪唑[2,1- b]噻唑衍生物,用光谱技术对它们进行表征,并研究对神经胶质瘤 C6 癌细胞系的细胞毒性和凋亡作用。还研究了针对粘着斑激酶的体外抗癌活性。大多数化合物,特别是带有 3-oxo-1-tiya-4-azaspiro[4.5]decane 部分的衍生物,与参考药物顺铂相比,表现出更高或相当的活性。在螺噻唑烷酮环的第八位和第二位具有甲基、丙基、苯基部分的化合物显示出高的 FAK 抑制活性。此外,分子对接研究揭示了合成化合物的结合模式。揭示了与位于活性位点的氨基酸残基的关键相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号