当前位置:
X-MOL 学术
›
Phys. Status Solidi B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Nature of Chemical Bonds in the Tetragonal Polymorph of InTe: First-Principles-Based Topological Analysis
Physica Status Solidi (B) - Basic Solid State Physics ( IF 1.5 ) Pub Date : 2021-05-22 , DOI: 10.1002/pssb.202100072 Aleksey V. Kovalenko 1 , Andrei V. Bandura 1 , Dmitry D. Kuruch 1 , Sergey I. Lukyanov 1 , Robert A. Evarestov 1
Physica Status Solidi (B) - Basic Solid State Physics ( IF 1.5 ) Pub Date : 2021-05-22 , DOI: 10.1002/pssb.202100072 Aleksey V. Kovalenko 1 , Andrei V. Bandura 1 , Dmitry D. Kuruch 1 , Sergey I. Lukyanov 1 , Robert A. Evarestov 1
Affiliation
|
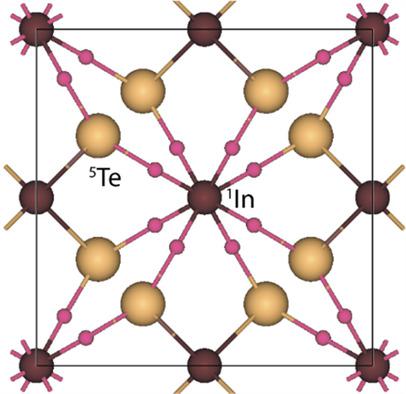
The topological analysis of the electron density is first performed for a bulk InTe crystal using the density functional theory calculations. Several types of two-center chemical interactions have been identified. Crystal orbital Hamilton population method is used to estimate the corresponding bond strength. As expected, the InTe chemical bonds in the –InTe2– ring chains turn out to be the strongest and have a noticeable covalent contribution. The InIn metallic bonds in linear –In– chains are much weaker. The results obtained reveal that the additional InTe bonds between the –In– and –InTe2– chains can be attributed to weak dative interactions. However, due to their multiplicity, these bonds can play an important role in the stability of the tetragonal InTe phase. The van der Waals interactions of neighboring –InTe2– chains also stabilize the crystal structure. Both Hirshfeld and Bader populations show that the effective charge of indium in the –InTe2– ring chain is noticeably greater than that in the –In– linear chain.
中文翻译:

InTe 四方多晶型物中化学键的性质:基于第一性原理的拓扑分析
首先使用密度泛函理论计算对块状 InTe 晶体进行电子密度的拓扑分析。已经确定了几种类型的双中心化学相互作用。晶体轨道哈密顿布居法用于估计相应的键强度。正如预期的那样,–InTe 2 – 环链中的 In Te 化学键被证明是最强的,并具有显着的共价贡献。线性-In-链中的In In 金属键要弱得多。获得的结果表明,–In– 和–InTe 2之间的额外 In Te 键– 链可以归因于弱与格相互作用。然而,由于它们的多样性,这些键可以在四方 InTe 相的稳定性中发挥重要作用。相邻–InTe 2 – 链的范德华相互作用也稳定了晶体结构。Hirshfeld 和 Bader 布居均表明,–InTe 2 – 环链中的铟有效电荷明显大于 –In– 线性链中的有效电荷。
更新日期:2021-05-22
中文翻译:

InTe 四方多晶型物中化学键的性质:基于第一性原理的拓扑分析
首先使用密度泛函理论计算对块状 InTe 晶体进行电子密度的拓扑分析。已经确定了几种类型的双中心化学相互作用。晶体轨道哈密顿布居法用于估计相应的键强度。正如预期的那样,–InTe 2 – 环链中的 In Te 化学键被证明是最强的,并具有显着的共价贡献。线性-In-链中的In In 金属键要弱得多。获得的结果表明,–In– 和–InTe 2之间的额外 In Te 键– 链可以归因于弱与格相互作用。然而,由于它们的多样性,这些键可以在四方 InTe 相的稳定性中发挥重要作用。相邻–InTe 2 – 链的范德华相互作用也稳定了晶体结构。Hirshfeld 和 Bader 布居均表明,–InTe 2 – 环链中的铟有效电荷明显大于 –In– 线性链中的有效电荷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号