Materials Today Physics ( IF 11.5 ) Pub Date : 2021-05-20 , DOI: 10.1016/j.mtphys.2021.100443 Qiancheng Zhou , Wei Zhang , Minqiang Qiu , Ying Yu
|
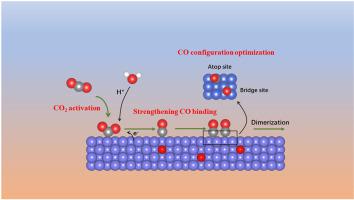
Among various studied catalysts, copper-based electrocatalysts can convert CO2 into hydrocarbons. Two main challenges in this research area are low selectivity, especially for value-added C2+ products and the high overpotentials required to initiate carbon dioxide reduction reaction (CO2RR). The interaction between copper and oxygen has always been inevitable in the design of copper-based catalysts owing to the intrinsic activity of copper and the oxygen-rich environment we live in. Recently, due to the unprecedented development of in-situ characterization, continuously increasing attention has been paid to oxygen species, which is usually accounting for the highest proportion of metalloid composition in copper-based catalysts. Herein, we have summarized the research progress on oxygen-containing copper catalysts, both experimentally and theoretically, and revealed the evolution of oxygen and the relationship between catalytic performance and oxygen species. It is found that the existence of subsurface oxygen in the electrochemical interface of copper-based electrodes has been proved both by in-situ characterization and theoretical simulation, which is also essential for efficient CO2RR to C2+ products. Additionally, selectivity to C2+ products is basically boosted in three ways: facilitating the conversion of CO2 from physical adsorption to chemical adsorption, strengthening intermediate binding and optimizing the adsorption configuration of CO2. It is expected that this review will provide clue for utilizing oxygen to enhance C2+ selectivity in the design of copper-based catalysts.
中文翻译:

氧气在二氧化碳电化学还原铜基催化剂中的作用
在各种研究的催化剂中,铜基电催化剂可以将 CO 2转化为碳氢化合物。该研究领域的两个主要挑战是选择性低,尤其是对于高附加值的 C2+ 产品以及引发二氧化碳还原反应(CO 2RR)。由于铜的固有活性和我们生活的富氧环境,铜和氧之间的相互作用在铜基催化剂的设计中一直是不可避免的。最近,由于原位表征的空前发展,不断增加氧物种已受到关注,氧物种通常占铜基催化剂中非金属成分的最高比例。在此,我们从实验和理论两个方面总结了含氧铜催化剂的研究进展,揭示了氧的演化以及催化性能与氧物种之间的关系。2 RR 到 C2+ 产品。此外,对C2+产物的选择性基本上通过三种方式提高:促进CO 2从物理吸附向化学吸附的转化、加强中间结合和优化CO 2的吸附构型。预计该综述将为利用氧气提高铜基催化剂设计中的 C2+ 选择性提供线索。



























 京公网安备 11010802027423号
京公网安备 11010802027423号