当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemically Mediated CO2 Capture Using Aqueous Cu(II)/Cu(I) Imidazole Complexes
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-05-19 , DOI: 10.1021/acsestengg.1c00068 Jonathan Boualavong 1 , Christopher A. Gorski 1
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-05-19 , DOI: 10.1021/acsestengg.1c00068 Jonathan Boualavong 1 , Christopher A. Gorski 1
Affiliation
|
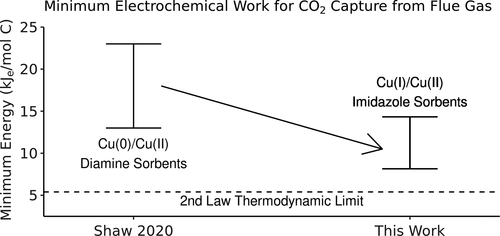
A major goal of developing electrochemical CO2 capture technologies is to minimize the energy demand. One strategy for decreasing energy demands of electrochemical capture technologies is increasing the ratio of CO2 molecules captured per transferred electron. Here, we examined an electrochemical capture approach that has the potential to capture up to two CO2 molecules per electron, which is higher than many existing approaches. We used the Cu(II)/Cu(I) redox couple to control the aqueous availability of a CO2 sorbent, 1,2-dimethylimidazole (Me2Im), by transitioning between Cu(Me2Im)4(aq)2+ and Cu(Me2Im)2(aq)+. As expected from equilibrium calculations, a solution containing reduced Cu(I) had a greater CO2 capacity than the oxidized Cu(II) state. In a bench-scale test, the energy demand for CO2 capture was 27 ± 6 kJe/mol C, despite operating at 7–11% energy efficiency due to a high experimentally-set cell voltage. We estimate that under market-ready concentration conditions and the same low energy efficiency, the energy demand will be approximately 65 ± 14 kJe/mol C, although it can only remove 60% of the CO2 from coal power plant flue gas (PCO2 = 0.15 atm) at equilibrium. To address this issue, we used an equilibrium model of the relevant chemical reactions to identify how altering the substituent groups on imidazole will influence the CO2 capture capacity and energy demand.
中文翻译:

使用 Cu(II)/Cu(I) 咪唑配合物进行电化学介导的 CO 2捕获
开发电化学CO 2捕获技术的主要目标是最小化能量需求。降低电化学捕获技术的能量需求的一种策略是增加每个转移电子捕获的CO 2分子的比率。在这里,我们研究了一种电化学捕获方法,该方法有可能每个电子捕获多达两个 CO 2分子,这高于许多现有方法。我们使用 Cu(II)/Cu(I) 氧化还原对通过在 Cu(Me 2 Im) 4(aq) 2之间转换来控制 CO 2吸附剂、1,2-二甲基咪唑 (Me 2 Im)的水溶液可用性+和 Cu(Me 2 Im)2(aq) +。正如平衡计算所预期的那样,含有还原 Cu(I) 的溶液比氧化 Cu(II) 状态具有更大的 CO 2容量。在实验室规模的测试中,尽管由于实验设置的电池电压高而以 7-11% 的能源效率运行,但CO 2捕获的能量需求为27 ± 6 kJ e /mol C。我们估计,在市场就绪浓度条件和同样低能效的情况下,能源需求将约为 65±14 kJ e /mol C,尽管它只能从煤电厂烟气中去除 60%的 CO 2 ( P CO 2= 0.15 atm) 处于平衡状态。为了解决这个问题,我们使用相关化学反应的平衡模型来确定改变咪唑上的取代基将如何影响 CO 2捕获能力和能量需求。
更新日期:2021-07-09
中文翻译:

使用 Cu(II)/Cu(I) 咪唑配合物进行电化学介导的 CO 2捕获
开发电化学CO 2捕获技术的主要目标是最小化能量需求。降低电化学捕获技术的能量需求的一种策略是增加每个转移电子捕获的CO 2分子的比率。在这里,我们研究了一种电化学捕获方法,该方法有可能每个电子捕获多达两个 CO 2分子,这高于许多现有方法。我们使用 Cu(II)/Cu(I) 氧化还原对通过在 Cu(Me 2 Im) 4(aq) 2之间转换来控制 CO 2吸附剂、1,2-二甲基咪唑 (Me 2 Im)的水溶液可用性+和 Cu(Me 2 Im)2(aq) +。正如平衡计算所预期的那样,含有还原 Cu(I) 的溶液比氧化 Cu(II) 状态具有更大的 CO 2容量。在实验室规模的测试中,尽管由于实验设置的电池电压高而以 7-11% 的能源效率运行,但CO 2捕获的能量需求为27 ± 6 kJ e /mol C。我们估计,在市场就绪浓度条件和同样低能效的情况下,能源需求将约为 65±14 kJ e /mol C,尽管它只能从煤电厂烟气中去除 60%的 CO 2 ( P CO 2= 0.15 atm) 处于平衡状态。为了解决这个问题,我们使用相关化学反应的平衡模型来确定改变咪唑上的取代基将如何影响 CO 2捕获能力和能量需求。











































 京公网安备 11010802027423号
京公网安备 11010802027423号