当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Noble Metal-Free FeOOH/Li0.1WO3 Core–Shell Nanorods for Selective Oxidation of Methane to Methanol with Visible–NIR Light
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-05-18 , DOI: 10.1021/acs.est.1c01152 Yi Zeng 1 , Xin Luo 2 , Feng Li 3 , Anhua Huang 1 , Hongmei Wu 3 , Guo Qin Xu 4 , Song Ling Wang 1, 5
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-05-18 , DOI: 10.1021/acs.est.1c01152 Yi Zeng 1 , Xin Luo 2 , Feng Li 3 , Anhua Huang 1 , Hongmei Wu 3 , Guo Qin Xu 4 , Song Ling Wang 1, 5
Affiliation
|
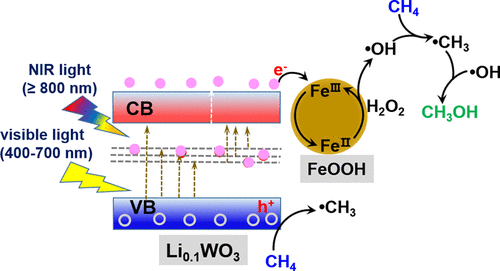
Hydroxyl radicals (•OH) generated in the photocatalytic process are crucial to the conversion of methane (CH4) to value-added methanol (CH3OH) at room temperature. However, utilizing noble metal-free catalysts and low-energy photons of solar light, such as visible and near-infrared light (vis–NIR), is difficult to provide more electron states to form •OH radicals. Here, we developed FeOOH/Li0.1WO3 core–shell nanorods via a two-step in/out co-modification of hexagonal tungsten oxide (h-WO3): (1) lithium ions intercalating into the hexagonal tunnels of h-WO3 to form Li0.1WO3 nanorods and (2) using FeOOH-wrapped Li0.1WO3 to obtain FeOOH/Li0.1WO3 core–shell nanorods. Introduction of lithium induces polaron transition in Li0.1WO3, enabling the absorption of vis-NIR light. Interestingly, FeOOH-based Fenton-like reaction when H2O2 is selected as an oxidant favors the generation of more •OH radicals available for CH4 oxidation to CH3OH. Meanwhile, FeOOH with FeIII as an “electron sink” highly improves the separation of photoinduced electrons and holes in Li0.1WO3. Eventually, efficient selective formation of CH4OH is achieved with remarkable generation rates up to ∼342 and ∼160 μmol g–1 at visible light (420–700 nm) and NIR light (≥800 nm), respectively. Our finding opens up new possibilities for developing noble metal-free catalysts for solar energy-driven CH4 conversion to CH3OH under ambient conditions.
中文翻译:

无贵金属 FeOOH/Li 0.1 WO 3核壳纳米棒,用于用可见光-近红外光选择性氧化甲烷为甲醇
光催化过程中产生的羟基 ( • OH) 对于在室温下将甲烷 (CH 4 )转化为具有附加值的甲醇 (CH 3 OH)至关重要。然而,利用不含贵金属的催化剂和太阳光的低能光子,例如可见光和近红外光 (vis-NIR),难以提供更多的电子态以形成• OH 自由基。在这里,我们通过六方氧化钨 (h-WO 3 )的两步进/出共改性开发了 FeOOH/Li 0.1 WO 3核壳纳米棒:(1) 锂离子嵌入 h-WO 的六方隧道3形成Li 0.1 WO 3纳米棒和(2)使用 FeOOH 包裹的 Li 0.1 WO 3获得 FeOOH/Li 0.1 WO 3核壳纳米棒。锂的引入会在 Li 0.1 WO 3 中诱导极化子跃迁,从而能够吸收可见光-NIR 光。Interestingly, FeOOH-based Fenton-like reaction when H 2 O 2 is selected as an oxidant favors the generation of more • OH radicals available for CH 4 oxidation to CH 3 OH. 同时,FeOOH 与 Fe III作为“电子汇”极大地改善了 Li 0.1 WO 3中光生电子和空穴的分离. 最终,实现了 CH 4 OH 的高效选择性形成,在可见光(420-700 nm)和 NIR 光(≥800 nm)下分别具有高达 ~342 和 ~160 μmol g –1 的显着生成率。我们的发现为开发无贵金属催化剂在环境条件下用于太阳能驱动的 CH 4转化为 CH 3 OH开辟了新的可能性。
更新日期:2021-06-01
中文翻译:

无贵金属 FeOOH/Li 0.1 WO 3核壳纳米棒,用于用可见光-近红外光选择性氧化甲烷为甲醇
光催化过程中产生的羟基 ( • OH) 对于在室温下将甲烷 (CH 4 )转化为具有附加值的甲醇 (CH 3 OH)至关重要。然而,利用不含贵金属的催化剂和太阳光的低能光子,例如可见光和近红外光 (vis-NIR),难以提供更多的电子态以形成• OH 自由基。在这里,我们通过六方氧化钨 (h-WO 3 )的两步进/出共改性开发了 FeOOH/Li 0.1 WO 3核壳纳米棒:(1) 锂离子嵌入 h-WO 的六方隧道3形成Li 0.1 WO 3纳米棒和(2)使用 FeOOH 包裹的 Li 0.1 WO 3获得 FeOOH/Li 0.1 WO 3核壳纳米棒。锂的引入会在 Li 0.1 WO 3 中诱导极化子跃迁,从而能够吸收可见光-NIR 光。Interestingly, FeOOH-based Fenton-like reaction when H 2 O 2 is selected as an oxidant favors the generation of more • OH radicals available for CH 4 oxidation to CH 3 OH. 同时,FeOOH 与 Fe III作为“电子汇”极大地改善了 Li 0.1 WO 3中光生电子和空穴的分离. 最终,实现了 CH 4 OH 的高效选择性形成,在可见光(420-700 nm)和 NIR 光(≥800 nm)下分别具有高达 ~342 和 ~160 μmol g –1 的显着生成率。我们的发现为开发无贵金属催化剂在环境条件下用于太阳能驱动的 CH 4转化为 CH 3 OH开辟了新的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号