Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-05-18 , DOI: 10.1016/j.jcou.2021.101576 Sachin Kumar Sharma , Arghya Banerjee , Bappi Paul , Mukesh Kumar Poddar , Takehiko Sasaki , Chanchal Samanta , Rajaram Bal
|
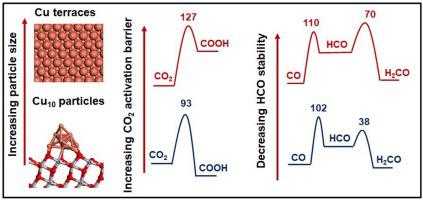
The hydrogenation of CO2 to methanol over Cu-nanoparticles supported on TiO2 nanocrystals was studied at 30 bar pressure and 200−300 °C. 5 wt% Cu-TiO2 catalyst was synthesized by a modified hydrothermal method (Cu-TiO2HT) and by incipient wetness impregnation method (Cu-TiO2IMP). TEM analysis of the Cu-TiO2HT catalyst revealed the formation of Cu-nanoparticles (3-5 nm), while larger Cu particle sizes were observed on the Cu-TiO2IMP catalyst. The Cu-TiO2HT catalyst showed superior catalytic activity (CO2 conversion ∼ 9.4 %) and methanol selectivity (∼ 96 %) at 200 °C and 30 bar pressure. Low CO2 conversions (∼6%) and high CO selectivity (∼40 %) was obtained on the Cu-TiO2IMP catalyst. Density functional theory (DFT) calculations indicated the CO2 activation to methanol to proceed via a reverse water gas shift pathway with a significantly lower (93 kJ/mol) CO2 activation barrier on the Cu-nanoparticles, relative to the larger Cu particles (127 kJ/mol). In addition, the higher selectivity towards methanol over the Cu-TiO2HT catalyst was attributed to the higher CO and HCO stability on the Cu nanoparticles. Time of stream (TOS) study of the Cu-TiO2 catalysts showed no significant deactivation even after 150 h with molar feed ratio 1:3:1 (CO2:H2: N2) at 200 °C.
中文翻译:

实验与计算研究相结合,揭示了Cu / TiO 2催化剂将CO 2加氢制甲醇的影响因素
研究了在30 bar的压力和200-300°C的条件下,负载在TiO 2纳米晶体上的Cu-纳米颗粒上CO 2加氢成甲醇的过程。通过改进的水热法(Cu-TiO 2 HT)和初湿浸渍法(Cu-TiO 2 IMP)合成了5 wt%的Cu-TiO 2催化剂。Cu-TiO 2 HT催化剂的TEM分析表明形成了铜纳米粒子(3-5纳米),而在Cu-TiO 2 IMP催化剂上观察到了较大的Cu粒径。Cu-TiO 2 HT催化剂显示出优异的催化活性(CO 2在200°C和30 bar的压力下,甲醇转化率约为9.4%),甲醇选择性约为96%。在Cu-TiO 2 IMP催化剂上获得了低的CO 2转化率(〜6%)和高的CO选择性(〜40%)。密度泛函理论(DFT)计算指示的CO 2活化甲醇经由与显著较低(93千焦/摩尔)CO反向水煤气变换途径进行2活化在Cu纳米颗粒屏障,相对于较大的Cu粒子( 127 kJ / mol)。另外,与Cu-TiO 2 HT催化剂相比,对甲醇的更高的选择性归因于对Cu纳米颗粒的更高的CO和HCO稳定性。Cu-TiO 2的流时间(TOS)研究即使在200℃下进料摩尔比为1:3:1(CO 2:H 2:N 2)的150小时后,催化剂也没有显示出明显的失活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号