当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bis(2-nitrophenyl) selenide, bis(2-aminophenyl) selenide and bis(2-aminophenyl) telluride: structural and theoretical analysis
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2021-05-17 , DOI: 10.1107/s2053229621005015 Raju Saravanan , Harkesh B. Singh , Ray J. Butcher
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2021-05-17 , DOI: 10.1107/s2053229621005015 Raju Saravanan , Harkesh B. Singh , Ray J. Butcher
|
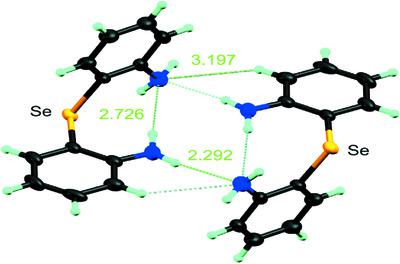
Three organoselenium and organotellurium compounds containing ortho substitutents, namely, bis(2-nitrophenyl) selenide, C12H8N2O4Se, 2, bis(2-aminophenyl) selenide, C12H12N2Se, 3, and bis(2-aminophenyl) telluride, C12H12N2Te, 7, have been investigated by both structural and theoretical methods. In the structures of all three compounds, there are intramolecular contacts between both Se and Te with the ortho substituents. In the case of 2, this is achieved by rotation of the nitro group from the arene plane. For 3, both amino groups exhibit pyramidal geometry and are involved in intramolecular N—H…Se interactions, with one also participating in intermolecular N—H…N hydrogen bonding. While 3 and 7 are structurally similar, there are some significant differences. In addition to both intramolecular N—H…Te interactions and intermolecular N—H…N hydrogen bonding, 7 also exhibits intramolecular N—H…N hydrogen bonding. In the packing of these molecules, for 2, there are weak intermolecular C—H…O contacts and these, along with the O…N interactions mentioned above, link the molecules into a three-dimensional array. For 3, in addition to the N—H…N and N—H…Se interactions, there are also weak intermolecular C—H…Se interactions, which also link the molecules into a three-dimensional array. On the other hand, 7 shows intermolecular N—H…N interactions linking the molecules into R22(16) centrosymmetric dimers. In the theoretical studies, for compound 2, AIM (atoms in molecules) analysis revealed critical points in the Se…O interactions with values of 0.017 and 0.026 a.u. These values are suggestive of weak interactions present between Se and O atoms. For 3 and 7, the molecular structures displayed intramolecular, as well as intermolecular, hydrogen-bond interactions of the N—H…N type. The strength of this hydrogen-bond interaction was calculated by AIM analysis. Here, the intermolecular (N—H…N) hydrogen bond is stronger than the intramolecular hydrogen bond. This was confirmed by the electron densities for 3 and 7 [ρ(r) = 0.015 and 0.011, respectively].
中文翻译:

双(2-硝基苯基)硒化物、双(2-氨基苯基)硒化物和双(2-氨基苯基)碲化物:结构和理论分析
三种含有邻位取代基的有机硒和有机碲化合物,即双(2-硝基苯基)硒化物、C 12 H 8 N 2 O 4 Se, 2、双(2-氨基苯基)硒化物、C 12 H 12 N 2 Se, 3和双(2-氨基苯基)碲化物,C 12 H 12 N 2 Te, 7已经通过结构和理论方法进行了研究。在所有三种化合物的结构中,Se 和 Te 之间都存在与邻位取代基的分子内接触。在2的情况下,这是通过从芳烃平面旋转硝基来实现的。对于3,两个氨基都表现出锥体几何形状,并参与分子内 N-H…Se 相互作用,其中一个还参与分子间 N-H…N 氢键。虽然3和7在结构上相似,但存在一些显着差异。除了分子内 N-H...Te 相互作用和分子间 N-H...N 氢键之外,7还表现出分子内 N-H...N 氢键。在这些分子的堆积中,对于2,存在弱的分子间 C-H…O 接触,这些与上述 O…N 相互作用一起,将分子连接成一个三维阵列。为3, 除了 N—H…N 和 N—H…Se 相互作用外,还有弱的分子间 C—H…Se 相互作用,它们也将分子连接成一个三维阵列。另一方面,7显示了将分子连接成R 2 2 (16) 中心对称二聚体的分子间 N-H…N 相互作用。在理论研究中,对于化合物2,AIM(分子中的原子)分析揭示了 Se…O 相互作用中的临界点,值为 0.017 和 0.026 au。这些值表明 Se 和 O 原子之间存在弱相互作用。对于3和7,分子结构显示出分子内以及分子间的 N-H…N 型氢键相互作用。这种氢键相互作用的强度是通过 AIM 分析计算的。这里,分子间(NH…N)氢键比分子内氢键强。3和7的电子密度证实了这一点[ρ ( r ) = 0.015 和 0.011,分别]。
更新日期:2021-06-05
中文翻译:

双(2-硝基苯基)硒化物、双(2-氨基苯基)硒化物和双(2-氨基苯基)碲化物:结构和理论分析
三种含有邻位取代基的有机硒和有机碲化合物,即双(2-硝基苯基)硒化物、C 12 H 8 N 2 O 4 Se, 2、双(2-氨基苯基)硒化物、C 12 H 12 N 2 Se, 3和双(2-氨基苯基)碲化物,C 12 H 12 N 2 Te, 7已经通过结构和理论方法进行了研究。在所有三种化合物的结构中,Se 和 Te 之间都存在与邻位取代基的分子内接触。在2的情况下,这是通过从芳烃平面旋转硝基来实现的。对于3,两个氨基都表现出锥体几何形状,并参与分子内 N-H…Se 相互作用,其中一个还参与分子间 N-H…N 氢键。虽然3和7在结构上相似,但存在一些显着差异。除了分子内 N-H...Te 相互作用和分子间 N-H...N 氢键之外,7还表现出分子内 N-H...N 氢键。在这些分子的堆积中,对于2,存在弱的分子间 C-H…O 接触,这些与上述 O…N 相互作用一起,将分子连接成一个三维阵列。为3, 除了 N—H…N 和 N—H…Se 相互作用外,还有弱的分子间 C—H…Se 相互作用,它们也将分子连接成一个三维阵列。另一方面,7显示了将分子连接成R 2 2 (16) 中心对称二聚体的分子间 N-H…N 相互作用。在理论研究中,对于化合物2,AIM(分子中的原子)分析揭示了 Se…O 相互作用中的临界点,值为 0.017 和 0.026 au。这些值表明 Se 和 O 原子之间存在弱相互作用。对于3和7,分子结构显示出分子内以及分子间的 N-H…N 型氢键相互作用。这种氢键相互作用的强度是通过 AIM 分析计算的。这里,分子间(NH…N)氢键比分子内氢键强。3和7的电子密度证实了这一点[ρ ( r ) = 0.015 和 0.011,分别]。











































 京公网安备 11010802027423号
京公网安备 11010802027423号