当前位置:
X-MOL 学术
›
Isr. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclization Strategies for the Concurrent Installation of Multiple Quaternary Stereogenic Centers
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2021-05-16 , DOI: 10.1002/ijch.202100014 Georgios Alachouzos 1 , Alison J. Frontier 2
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2021-05-16 , DOI: 10.1002/ijch.202100014 Georgios Alachouzos 1 , Alison J. Frontier 2
Affiliation
|
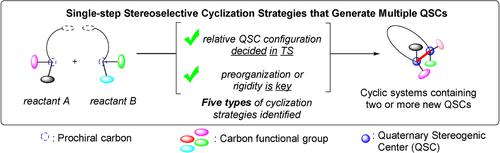
In this review we highlight the general cyclization strategies currently available to organic chemists for the concurrent and stereoselective installation of multiple Quaternary Stereogenic Centers (QSC) atoms in cyclic or polycyclic architectures. QSCs embedded in rigid cyclic architectures are motifs found in many blockbuster drugs and important bioactive natural product classes, and yet, direct access to these structures stereoselectively from simple precursors remains a significant challenge. Underscoring the difficulty associated with their synthesis, such topologically three-dimensional molecules are underrepresented in existing small molecule compound libraries, which are instead dominated by linear or flat molecules. This review focuses on methods disclosed in both natural product synthesis and methodology studies since the turn of the 21st century. The cases to be examined successfully achieve these challenging transformations: (1) one-step assembly of the cyclized architecture; and (2) concurrent stereoselective installation of multiple (≥2) new QSCs. These cyclization strategies, which address the aforementioned fundamental challenges in complex molecule synthesis, have been categorized into five broad groups: i) Biomimetic Polyene Cyclization Cascades; ii) Cyclization Cascades of Prochiral Alkenes; iii) Cycloadditions; iv) Dearomatizations; v) Electrocyclizations.
中文翻译:

多个第四纪立体中心同时安装的环化策略
在这篇综述中,我们重点介绍了有机化学家目前可用于同时和立体选择性安装多个第四纪立体中心的一般环化策略(QSC) 环状或多环结构中的原子。嵌入刚性环状结构的 QSC 是许多重磅药物和重要的生物活性天然产物类别中发现的基序,然而,从简单的前体立体选择性地直接访问这些结构仍然是一个重大挑战。强调与它们合成相关的困难,这种拓扑三维分子在现有的小分子化合物库中代表性不足,而是由线性或扁平分子主导。这次审查的重点,因为在21之交在自然产物合成和方法论研究公开的方法ST世纪。要检查的案例成功地实现了这些具有挑战性的转变:(1)循环架构的一步组装; (2)同时立体选择性安装多个 (≥2) 新 QSC。这些环化策略解决了上述复杂分子合成中的基本挑战,可分为五个大组:i) 仿生多烯环化级联;ii) 前手性烯烃的环化级联;iii) 环加成;iv) 脱芳构化;v) 电环化。
更新日期:2021-05-16
中文翻译:

多个第四纪立体中心同时安装的环化策略
在这篇综述中,我们重点介绍了有机化学家目前可用于同时和立体选择性安装多个第四纪立体中心的一般环化策略(QSC) 环状或多环结构中的原子。嵌入刚性环状结构的 QSC 是许多重磅药物和重要的生物活性天然产物类别中发现的基序,然而,从简单的前体立体选择性地直接访问这些结构仍然是一个重大挑战。强调与它们合成相关的困难,这种拓扑三维分子在现有的小分子化合物库中代表性不足,而是由线性或扁平分子主导。这次审查的重点,因为在21之交在自然产物合成和方法论研究公开的方法ST世纪。要检查的案例成功地实现了这些具有挑战性的转变:(1)循环架构的一步组装; (2)同时立体选择性安装多个 (≥2) 新 QSC。这些环化策略解决了上述复杂分子合成中的基本挑战,可分为五个大组:i) 仿生多烯环化级联;ii) 前手性烯烃的环化级联;iii) 环加成;iv) 脱芳构化;v) 电环化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号