Science of the Total Environment ( IF 8.2 ) Pub Date : 2021-05-15 , DOI: 10.1016/j.scitotenv.2021.147780 Gaolong Zhong 1 , Fang Wan 1 , Juan Lan 1 , Xuanxuan Jiang 1 , Shaofeng Wu 1 , Jiaqiang Pan 1 , Zhaoxin Tang 1 , Lianmei Hu 1
|
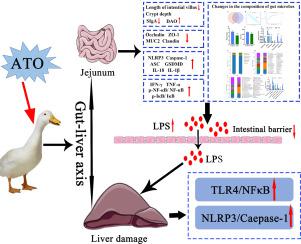
Arsenic is an important hazardous metalloid commonly found in polluted soil, rivers and groundwater. However, few studies exist regarding the effect of arsenic trioxide (ATO) on the gut-liver axis and consequent hepatotoxicity in waterfowl. Here, we investigated the influence of ATO on duck intestines and livers, and explored the role of the gut-liver axis in ATO-induced hepatotoxicity and intestinal toxicity. Our results demonstrated that ATO-exposure induced intestinal damage, liver inflammatory cell infiltration and vesicle steatosis. Additionally, the intestinal microbiota community in ATO-exposed ducks displayed significantly decreased α-diversity and an altered bacterial composition. Moreover, ATO-exposure markedly reduced the expression of intestinal barrier-related proteins (Claudin-1, MUC2, ZO-1 and Occludin), resulting in increased intestinal permeability and elevated lipopolysaccharide levels. Simultaneously, ATO-exposure also upregulated pyroptosis-related index levels in the liver and jejunum, and increased pro-inflammatory cytokine production (IFN-γ, TNF-α, IL-18, and IL-1β). Our further mechanistic studies showed that ATO-induced liver and jejunum inflammation were provoked by the activation of the LPS/TLR4/NF-κB signaling pathway and NLRP3 inflammasome. In summary, these results manifested that ATO exposure can cause liver and jejunal inflammation and pyroptosis, and the indirect gut-liver axis pathway may play an essential role in the potential mechanism of ATO-induced hepatotoxicity.
中文翻译:

砷暴露会引起肠屏障损伤,进而激活肠肝轴,从而导致鸭子的肝脏发炎和肝细胞凋亡
砷是一种重要的有害准金属,通常在污染的土壤,河流和地下水中发现。但是,关于三氧化二砷(ATO)对肠肝轴的影响以及随之而来的水禽肝毒性的研究很少。在这里,我们调查了ATO对鸭肠和肝脏的影响,并探讨了肠肝轴在ATO诱导的肝毒性和肠毒性中的作用。我们的研究结果表明,ATO暴露可引起肠损伤,肝炎性细胞浸润和囊泡脂肪变性。此外,暴露于ATO的鸭子中的肠道菌群群落显着降低了α多样性并改变了细菌组成。此外,ATO暴露显着降低了肠屏障相关蛋白(Claudin-1,MUC2,ZO-1和Occludin)的表达,导致肠道通透性增加和脂多糖水平升高。同时,ATO暴露还上调了肝脏和空肠中与凋亡相关的指数水平,并增加了促炎性细胞因子的产生(IFN-γ,TNF-α,IL-18和IL-1β)。我们进一步的机理研究表明,LPS / TLR4 /NF-κB信号通路和NLRP3炎性小体的激活引起了ATO引起的肝脏和空肠炎症。总之,这些结果表明,ATO暴露可引起肝脏和空肠炎症和细胞凋亡,间接的肠-肝轴途径可能在ATO诱导的肝毒性的潜在机制中起重要作用。并增加促炎细胞因子的产生(IFN-γ,TNF-α,IL-18和IL-1β)。我们进一步的机理研究表明,LPS / TLR4 /NF-κB信号通路和NLRP3炎性小体的激活引起了ATO引起的肝脏和空肠炎症。总而言之,这些结果表明,ATO暴露可引起肝脏和空肠炎症和细胞凋亡,并且间接的肠-肝轴途径可能在ATO诱导的肝毒性的潜在机制中起着至关重要的作用。并增加促炎细胞因子的产生(IFN-γ,TNF-α,IL-18和IL-1β)。我们进一步的机理研究表明,LPS / TLR4 /NF-κB信号通路和NLRP3炎性小体的激活引起了ATO引起的肝脏和空肠炎症。总之,这些结果表明,ATO暴露可引起肝脏和空肠炎症和细胞凋亡,间接的肠-肝轴途径可能在ATO诱导的肝毒性的潜在机制中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号