NanoImpact ( IF 4.7 ) Pub Date : 2021-05-15 , DOI: 10.1016/j.impact.2021.100324 Archini Paruthi 1 , Jared M Brown 2 , Emila Panda 1 , Abhay Raj Singh Gautam 1 , Sanjay Singh 3 , Superb K Misra 1
|
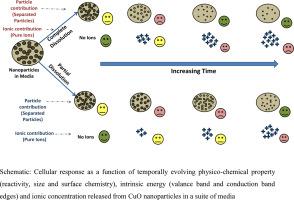
Nanoparticles under a reactive microenvironment have the propensity to undergo morphological and compositional changes, which can translate into band edge widening. Although cell membrane depolarization has been linked with the electronic band structure of nanomaterials in their native state, the change in band structure as a consequence of a soluble nanoparticle system is less studied. Therefore we studied the consequence of dissolution of CuO nanoparticles on the band structure and correlated it with its ability to induce intracellular oxidative stress. The temporal variation in bandgap, fermi energy level and valence band maxima were evaluated on the remnant CuO nanoparticles post dissolution. CuO nanoparticles showed a very high dissolution in simulated body fluid (51%) and cell culture media (75%). This dissolution resulted in an in situ physico-chemical transformation of CuO nanoparticles. A temporal increase in the bandgap energy as a result of media interaction was up to 107%. Temporal variation in the flat band potentials with the generation of intracellular ROS, cell viability, late and early apoptosis in addition to necrosis on RAW 264.7 cells was established due to biological redox potential overlap. The mRNA expression for TNF-α, IL-6, IL-1β and IL-10 in response to the particle treatment was also evalulated for 6 h. Through this study, we establish that the toxicological potential of CuO nanoparticles is a temporal function of band energies (its overlap with the intracellular redox potential) followed by release of ionic species in the cytotoxic regime.
中文翻译:

CuO 纳米粒子的能带能量随溶解度的变化及其对细胞反应的影响
反应性微环境下的纳米颗粒有发生形态和成分变化的倾向,这可以转化为带边展宽。尽管细胞膜去极化与纳米材料天然状态下的电子能带结构有关,但可溶性纳米粒子系统导致的能带结构变化的研究较少。因此,我们研究了 CuO 纳米粒子溶解对能带结构的影响,并将其与其诱导细胞内氧化应激的能力相关联。对溶解后残留的 CuO 纳米颗粒的带隙、费米能级和价带最大值的时间变化进行了评估。CuO 纳米颗粒在模拟体液 (51%) 和细胞培养基 (75%) 中表现出非常高的溶解度。这种溶解导致了CuO 纳米粒子的原位物理化学转化。由于介质相互作用,带隙能量暂时增加高达 107%。由于生物氧化还原电位重叠,平带电位随着细胞内 ROS 的产生、细胞活力、晚期和早期细胞凋亡以及 RAW 264.7 细胞的坏死而发生时间变化。还评估了 6 小时内响应颗粒处理的 TNF-α、IL-6、IL-1β 和 IL-10 的 mRNA 表达。通过这项研究,我们确定 CuO 纳米粒子的毒理学潜力是带能(其与细胞内氧化还原电位重叠)的时间函数,随后在细胞毒性状态下释放离子物质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号