Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From classical to supramolecular dynamic stereochemistry: Double crystallization-induced diastereomerization of thiazine sulfonamide
Chirality ( IF 2.8 ) Pub Date : 2021-05-15 , DOI: 10.1002/chir.23316 Olga A. Lodochnikova 1, 2 , Daria P. Gerasimova 1, 2 , Vitaly V. Plemenkov 2
Chirality ( IF 2.8 ) Pub Date : 2021-05-15 , DOI: 10.1002/chir.23316 Olga A. Lodochnikova 1, 2 , Daria P. Gerasimova 1, 2 , Vitaly V. Plemenkov 2
Affiliation
|
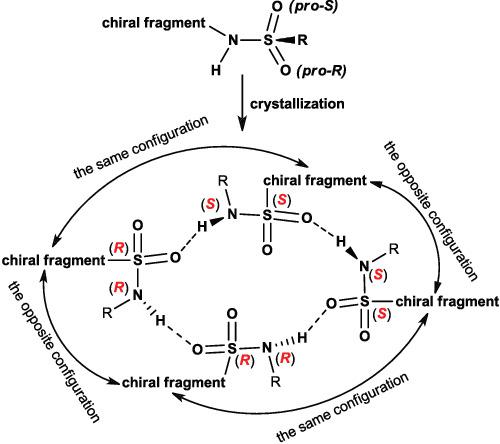
The interrelation between the configurational lability of nitrogen and sulfur atoms within the –NH–SO2 group of some thiazine sulfonamides is discussed. We have found that the compounds of the above series can crystallize as various diastereomers by the nitrogen atom, the relative configuration of the nitrogen atom determining the relative supramolecular configuration of the newly formed chiral sulfur atom. The paper presents a stereochemical transformation, which we have called “double crystallization-induced diastereomerization.”
中文翻译:

从经典到超分子动态立体化学:双结晶诱导的噻嗪磺酰胺非对映异构化
讨论了一些噻嗪磺酰胺的-NH-SO 2基团中氮和硫原子的构型不稳定性之间的相互关系。我们发现上述系列的化合物可以通过氮原子结晶为各种非对映异构体,氮原子的相对构型决定了新形成的手性硫原子的相对超分子构型。该论文介绍了一种立体化学转化,我们称之为“双结晶诱导的非对映异构化”。
更新日期:2021-06-08
中文翻译:

从经典到超分子动态立体化学:双结晶诱导的噻嗪磺酰胺非对映异构化
讨论了一些噻嗪磺酰胺的-NH-SO 2基团中氮和硫原子的构型不稳定性之间的相互关系。我们发现上述系列的化合物可以通过氮原子结晶为各种非对映异构体,氮原子的相对构型决定了新形成的手性硫原子的相对超分子构型。该论文介绍了一种立体化学转化,我们称之为“双结晶诱导的非对映异构化”。











































 京公网安备 11010802027423号
京公网安备 11010802027423号